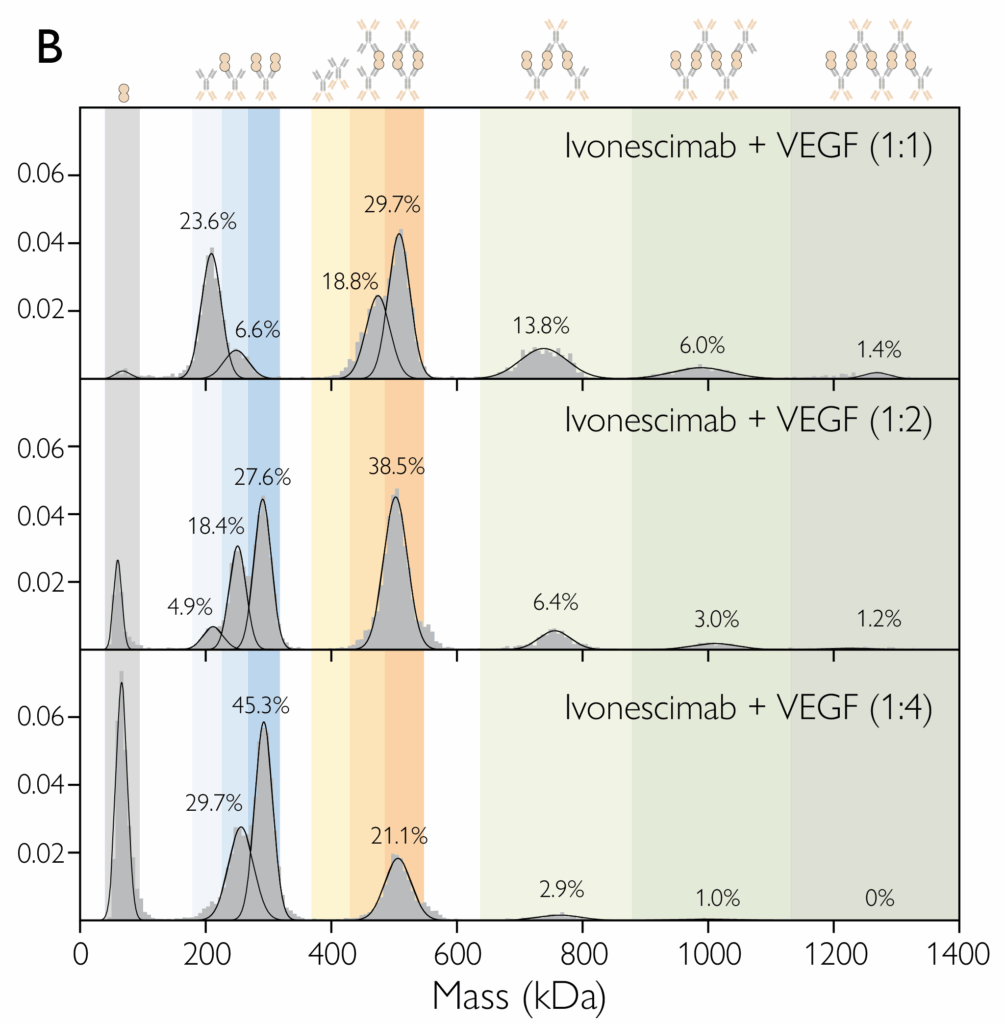
Mass photometry and macro mass photometry are powerful bioanalytical techniques that are being adopted across the life sciences. From academic researchers to CDMOs and leading biopharma companies, many have harnessed […]

USP employs mass photometry as key orthogonal method for empty/full characterization of AAV capsids Refeyn’s SamuxMP mass photometer for AAV analytics – used for characterizing the U.S. Pharmacopeia’s new adeno-associated […]

The new Refeyn brochure is your complete guide to mass photometry solutions and services This brochure includes: A brief explanation of how mass photometry and macro mass photometry work An […]
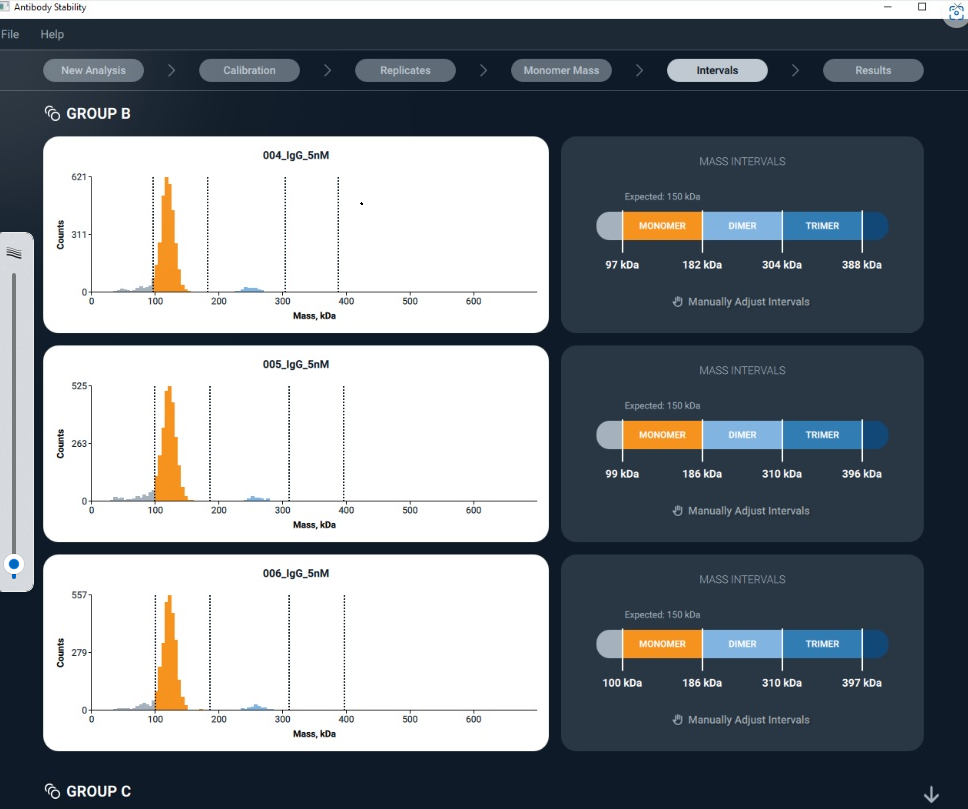
Refeyn has developed and launched a custom software platform providing automated analysis of large mass photometry data sets. Refeyn’s new StreamlineMP software suite offers specialist analysis modules, automating data […]
The importance of effective adeno-associated virus (AAV) analysis – and how mass photometry can support this – has been recognized in recent publications.





