Adeno-associated viruses (AAVs) have become one of the most commonly used vectors to deliver gene therapies. As their use grows, so does the need for accurate, efficient AAV analytics. Mass photometry can monitor sample critical quality attributes (CQAs) throughout downstream workflows – accelerating manufacturing and process development, to ultimately help better therapeutics reach patients faster.
Quantification of AAV empty/full ratio
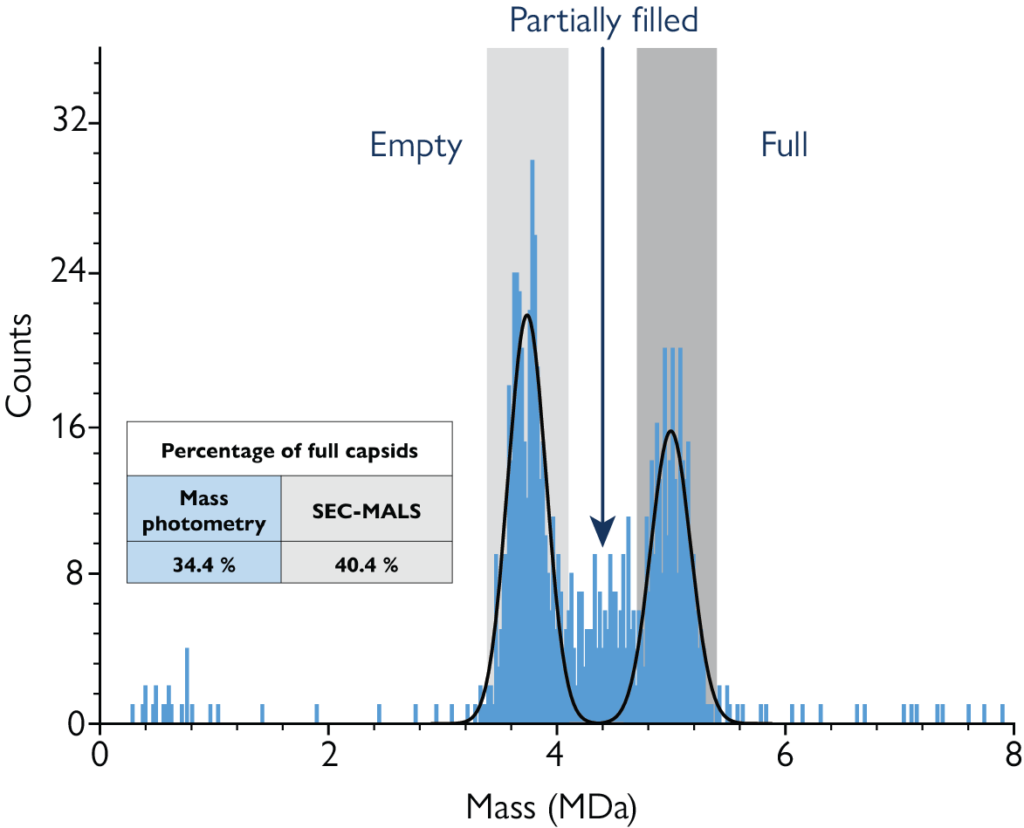
Mass photometry accurately measures empty/full AAV ratios, and resolves populations of partially filled and overfull capsids. It works by using light to measure the mass of each particle, which varies depending on the mass of the encapsidated genome.
Figure: Mass photometry resolves empty, full and partially filled AAV subpopulations. Here, populations of empty and full AAV vectors form clearly visible peaks and represent the bulk of the sample, while partially filled capsids are distributed across an intermediate mass range. The mass photometry measurement was consistent with a SEC-MALS measurement of the same sample.
Learn more about quantifying heterogeneous AAV populations
Agreement with orthogonal methods
Scientists working with AAVs must choose from among a range of options in the AAV vector analytics toolbox and it may be difficult to decide whether to prioritize accuracy or usability. With mass photometry, the difficulty disappears – mass photometers are highly accurate instruments that can be used over and over, throughout AAV purification processes
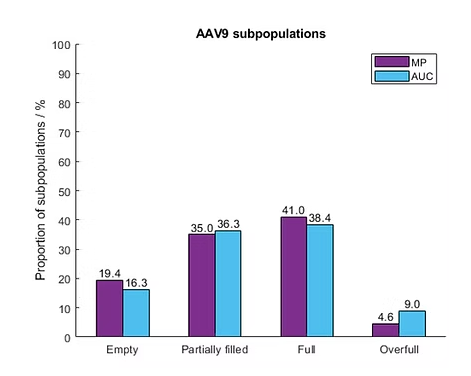
Mass photometry has been proven to provide comparable results to SEC-MALS, cryo-TEM and analytical ultracentrifugation (AUC) while offering important practical advantages, including speed, ease of use and suitability for use in a GMP-compliant environment
Figure: Mass photometry (MP) results for AAV empty/full analysis are comparable to those obtained using AUC, a commonly used technology for AAV characterization. Source: Wagner et al., Int. J. Mol Sci, 2023.
Read more on comparing analytical approaches for AAV characterization
Transforming analytical workflows for AAV purification
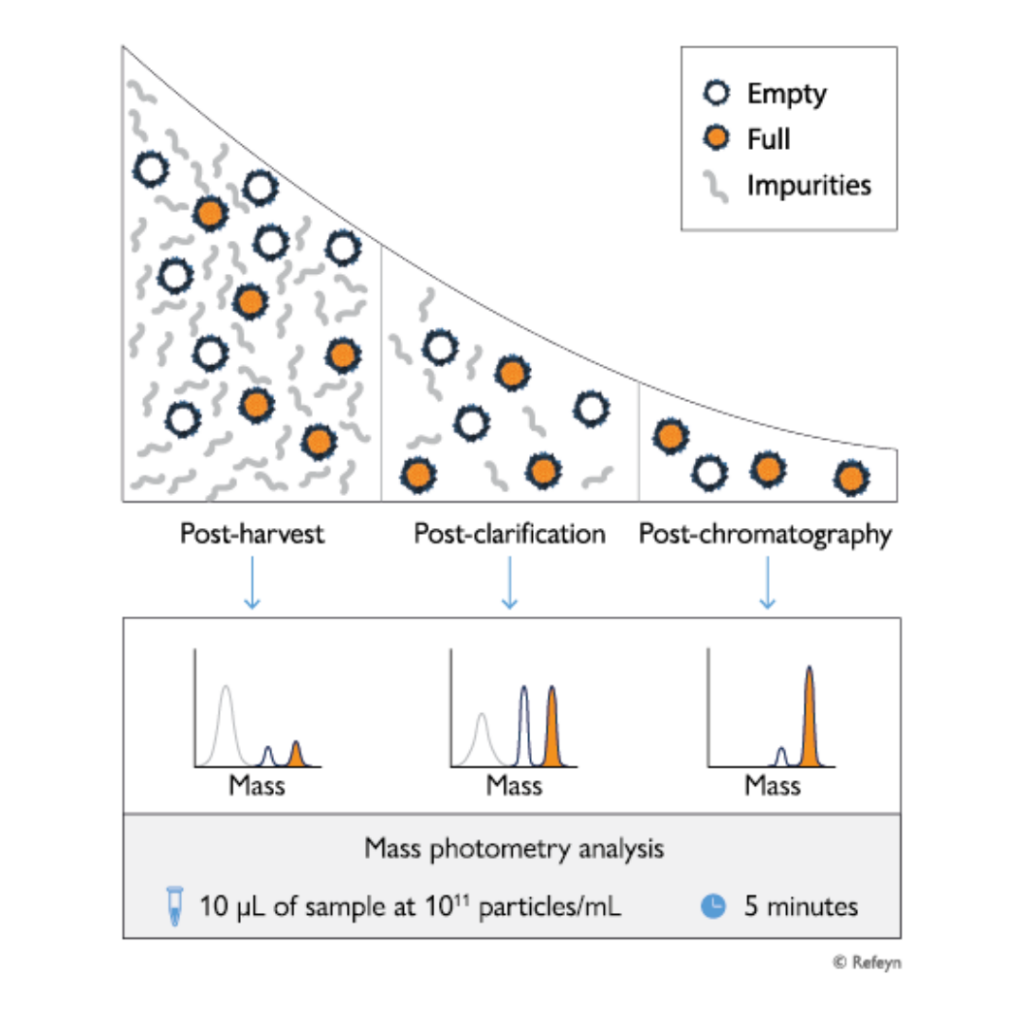
Results in minutes
Low sample requirement
Titer estimation
Benchtop instrument
Easy to use
Any serotype
Suitable for GMP-compliant environments
Combining speed, ease of use and low sample requirements, mass photometry integrates seamlessly into workflows for the downstream processing of AAVs in gene therapy development and manufacturing. It is also suitable for GMP-compliant environments.
Find out how mass photometry can speed up your AAV analytics workflows
Mass photometry solutions for AAV vector analytics: The SamuxMP product line
- SamuxMP
-

A mass photometer optimized for adeno-associated virus (AAV) characterization, the SamuxMP is an essential tool for AAV research, development and manufacturing. It features:
Characterization of AAV samples
Rapid analysis requiring little sample
User-friendly operation
- SamuxMP Auto
-

The SamuxMP Auto is the automated version of Refeyn’s solution for AAV characterization, autonomously measuring up to 24 samples in approximately 90 minutes.
- SamuxMP software package for GMP
-
The SamuxMP software package for GMP expands the use of the SamuxMP line of mass photometers to GMP-regulated environments. The software supports compliance with FDA 21 CFR 11 (US) and EU GMP Annex 11, which enables its use within AAV-based gene therapy development and manufacturing pipelines.
Read the white paper: Application of mass photometry in GMP-regulated environments for characterization of AAV samples.
- Refeyn consumables
-

By using Refeyn consumables, you can spend less time preparing for measurements and gain greater confidence in your data. The consumables range includes calibrants, sample carrier slides and more.
To streamline your AAV measurements, use MassFerence P2, Refeyn’s bespoke calibrant for the Samux line of mass photometers.

A mass photometer optimized for adeno-associated virus (AAV) characterization, the SamuxMP is an essential tool for AAV research, development and manufacturing. It features:
Characterization of AAV samples
Rapid analysis requiring little sample
User-friendly operation

The SamuxMP Auto is the automated version of Refeyn’s solution for AAV characterization, autonomously measuring up to 24 samples in approximately 90 minutes.

The SamuxMP software package for GMP expands the use of the SamuxMP line of mass photometers to GMP-regulated environments. The software supports compliance with FDA 21 CFR 11 (US) and EU GMP Annex 11, which enables its use within AAV-based gene therapy development and manufacturing pipelines.
Read the white paper: Application of mass photometry in GMP-regulated environments for characterization of AAV samples.

By using Refeyn consumables, you can spend less time preparing for measurements and gain greater confidence in your data. The consumables range includes calibrants, sample carrier slides and more.
To streamline your AAV measurements, use MassFerence P2, Refeyn’s bespoke calibrant for the Samux line of mass photometers.
Testimonials from mass photometry users
Eduard Ebberink
Researcher at Utrecht University
“We’re generating data to compare mass photometry with gold standards like AUC and cryoTEM. Early indications show that it’s much quicker and equally accurate. Partners who’ve compared mass photometry to other methods themselves vouch for its accuracy. It’s a game changer in AAV characterization, for us as well as our clients.”
Quentin Bazot
Process Development and Innovation Manager, ABL Europe
“I will try to keep mass photometry as integrated in my work as possible because it is a very honest technique that gives you a direct picture of your sample and there is not a lot of room for interpretation.”
Luca Schulz
graduate student at the Max Planck Institute for Terrestrial Microbiology in Marburg
“We could not only differentiate full and empty capsids, but also estimate the size of the encapsidated genome from the MP data. This result really showcases the capability of the MP technique.”
Grzegorz Piszczek
Director of the Biophysics Core Facility National Heart, Lung, and Blood Institute (NIH)
View further resources related to AAV analytics
- Selected publications
-
D. Wu, P. Hwang, T. Li, and G. Piszczek, “Rapid characterization of adeno-associated virus (AAV) gene therapy vectors by mass photometry,” Gene Ther., pp. 1–7, Jan. 2022, doi: 10.1038/s41434-021-00311-4 – [Link]
Ebberink EH, Ruisinger A, Nuebel M, Thomann M, Heck AJ. Assessing production variability in empty and filled adeno-associated viruses by single molecule mass analyses. Mol. Ther. Methods Clin. Dev. 2022 Dec 8;27:491-501.C. Wagner, F. F. Fuchsberger, B. – [Link]
Innthaler, M. Lemmerer, and R. Birner-Gruenberger, “Quantification of Empty, Partially Filled and Full Adeno-Associated Virus Vectors Using Mass Photometry,” Int. J. Mol. Sci., vol. 24, no. 13, Art. no. 13, Jan. 2023, doi: 10.3390/ijms241311033. – [Link]
Hiemenz C, Baumeister N, Helbig C, Hawe A, Babutzka S, Michalakis S, Friess W, Menzen T. Genome length determination in adeno-associated virus vectors with mass photometry. Mol. Ther. Methods Clin. Dev. 2023 Dec 14;31. – [Link]
- Webinars
-
Streamlining AAV characterization with automated mass photometry
Are Your AAVs Filled? Determine Rapidly with Mass Photometry
Assessing production variability in adeno-associated viruses by single-particle mass analysis
Harnessing mass photometry for AAV sample characterization in GMP-regulated environments
An Essential PAT Technology for Downstream Processing in AAV Manufacturing
- Brochures
-
- App and tech notes
-
- Refeyn posts
-
D. Wu, P. Hwang, T. Li, and G. Piszczek, “Rapid characterization of adeno-associated virus (AAV) gene therapy vectors by mass photometry,” Gene Ther., pp. 1–7, Jan. 2022, doi: 10.1038/s41434-021-00311-4 – [Link]
Ebberink EH, Ruisinger A, Nuebel M, Thomann M, Heck AJ. Assessing production variability in empty and filled adeno-associated viruses by single molecule mass analyses. Mol. Ther. Methods Clin. Dev. 2022 Dec 8;27:491-501.C. Wagner, F. F. Fuchsberger, B. – [Link]
Innthaler, M. Lemmerer, and R. Birner-Gruenberger, “Quantification of Empty, Partially Filled and Full Adeno-Associated Virus Vectors Using Mass Photometry,” Int. J. Mol. Sci., vol. 24, no. 13, Art. no. 13, Jan. 2023, doi: 10.3390/ijms241311033. – [Link]
Hiemenz C, Baumeister N, Helbig C, Hawe A, Babutzka S, Michalakis S, Friess W, Menzen T. Genome length determination in adeno-associated virus vectors with mass photometry. Mol. Ther. Methods Clin. Dev. 2023 Dec 14;31. – [Link]
Streamlining AAV characterization with automated mass photometry
Are Your AAVs Filled? Determine Rapidly with Mass Photometry
Assessing production variability in adeno-associated viruses by single-particle mass analysis
Harnessing mass photometry for AAV sample characterization in GMP-regulated environments
An Essential PAT Technology for Downstream Processing in AAV Manufacturing
