Streamlining adenoviral vector analysis with macro mass photometry
Macro mass photometry accurately distinguishes empty/full AdVs
Adenoviruses (AdVs) are being increasingly used as vectors in vaccines, gene therapies and cancer treatments. Key quality attributes, such as the purity of samples and the proportion of capsids containing the genomic load, need to be measured during therapeutic production. However, current analytical techniques are slow, expensive and not always easy to access. This creates a bottleneck in developing therapeutics and optimizing production and purification pipelines.
Macro mass photometry represents a significant advancement in adenoviral vector analysis. Its ability to provide rapid, accurate and label-free characterization of AdV particles makes it an invaluable tool for optimizing production and ensuring product safety. Discover how our technology can enhance your adenoviral vector analysis workflows.
On this Page
- Macro mass photometry quickly characterizes adenoviral vector samples
- Macro mass photometry accurately measures empty/full adenovirus proportions
- KaritroMP measurements are comparable to widely used orthogonal methods
- Macro mass photometry solutions for AdV analysis
- Testimonials from macro mass photometry users
- Additional resources
Macro mass photometry quickly characterizes adenoviral vector samples
Macro mass photometry is a novel technology developed for analyzing large viral vectors, such as AdVs, addressing analytical challenges in the development and production stages of cell and gene therapies and vaccines. For individual adenoviral vector particles, it simultaneously measures two distinct parameters: Size (particle diameter) and scattering contrast (a qualitative measure related to the particle’s composition and mass). This multiparametric measurement produces size-contrast distribution data that enables the differentiation of subpopulations within a sample, for example, particles with the same size but different mass.
The KaritroMP is a state-of-the-art benchtop instrument that utilizes the power of macro mass photometry, returning data within as little as six minutes, making it the fastest instrument for this analysis on the market.
Key benefits of macro mass photometry
- Speed: Provides results within minutes, drastically accelerating workflow efficiency and supporting timely decision-making.
- Simplicity: Label-free analysis eliminates the need for dyes and extensive sample preparation. Multiparameter measurements occur simultaneously.
- Versatility: Convenient to use at any processing step, to assess sample suitability for downstream processing.
- Cost-efficiency: Detects process faults early, saving time and money.
- Comprehensive analysis: Evaluates the purity of adenoviral vector samples and characterizes subpopulations, quickly providing accurate key analytical details.
Macro mass photometry accurately measures empty/full adenovirus proportions
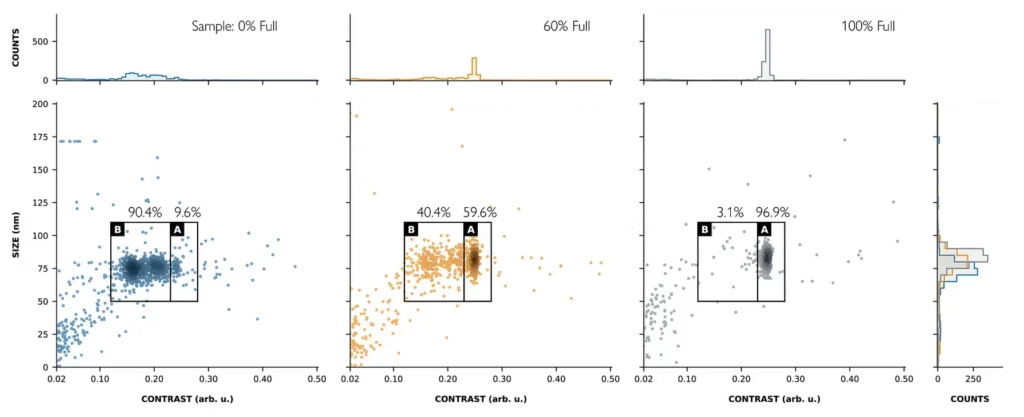
KaritroMP measurements are comparable to widely used orthogonal methods
Current adenoviral vector analytical techniques often trade accuracy for practicality. Traditional methods such as analytical ultracentrifugation (AUC) may be accurate, but are not fast or convenient, while faster, cheaper methods (for example, those using PCR) lack accuracy.
In collaboration with researchers at the University of Oxford, Jenner Institute, a comparison was conducted between AUC, negative stain electron microscopy (nsEM), Digital PCR : Anion exchange high-performance liquid chromatography (dPCR: AEX-HPLC), SDS denaturing (A260:A280), and macro mass photometry to evaluate their ability to accurately determine AdV capsid % full for a series of samples (Fig. 2).
Macro mass photometry can analyze samples with comparable accuracy to complex techniques like AUC and nsEM, but in minutes as opposed to hours, with minimal training and with low sample consumption. This makes macro mass photometry ideal for in-process evaluation of sample purity and capsid loading, streamlining adenovirus production and purification.
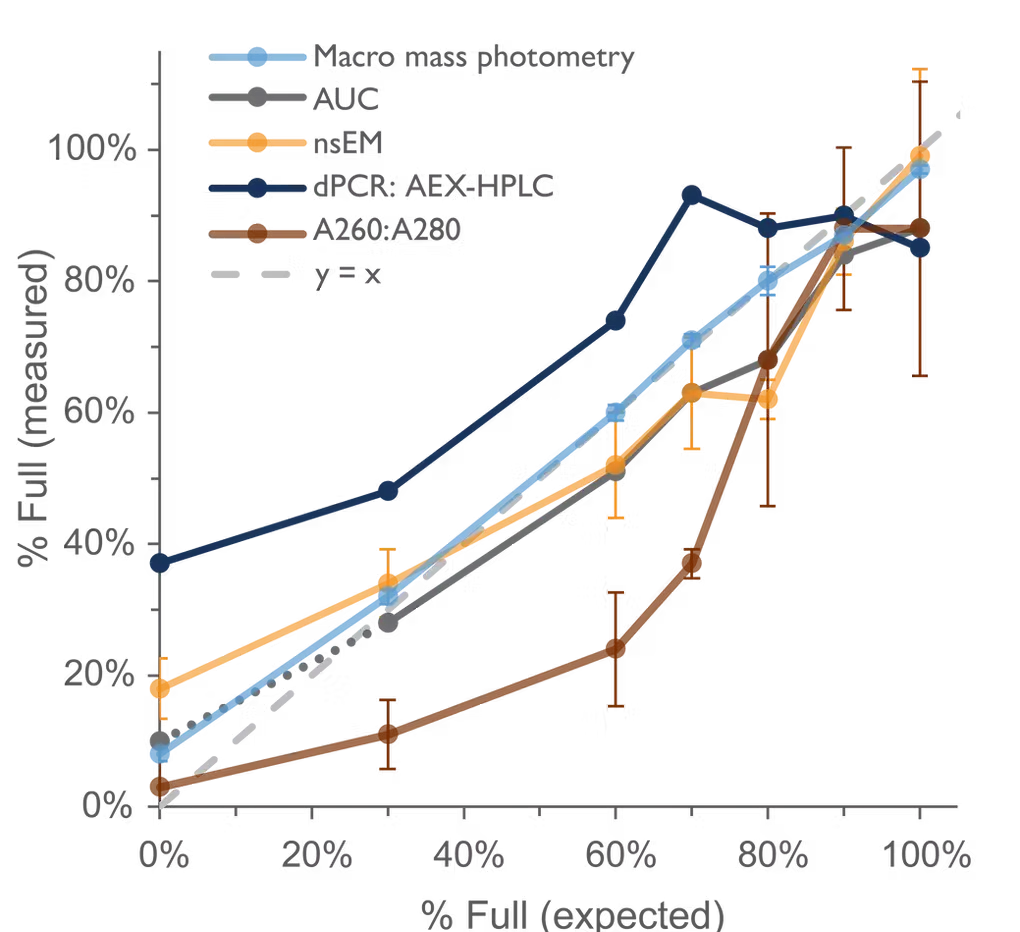
Fig. 2 Analysis of a series of AdV empty/full mixtures with macro mass photometry and a range of orthogonal techniques. Error bars are the SD of triplicate measurements for macro mass photometry, nsEM and A260:A280. Macro mass photometry, AUC and nsEM showed agreement with one another and with the expected values (y = x). Data were generated or obtained by the Jenner Institute (U. Oxford).
Macro mass photometry solutions for AdV analysis
- KaritroMP
-

The new KaritroMP is a benchtop instrument that characterizes large viral vectors. Ideal for informing process development in preclinical settings and early drug development, it analyzes samples of adenovirus vectors (AdV) and lentivirus vectors (LVV) in just minutes. The instrument offers essential vector analysis capabilities, including the assessment of sample purity and stability:
Fast, simple, qualitative AdV and LVV analysis
Differentiation of multiple populations, even those of the same size
Evaluation of relative changes across samples, from crude to purified
- MassGlass KV
-

The MassGlass KV sample carrier is an essential tool for rapid, consistent, and reproducible characterization of adenovirus and lentivirus vectors. Designed for use in a controlled environment, it enables the safe handling of BSL-2 samples.
- Macro mass photometry consumables
-
By using Refeyn consumables, you can spend less time preparing for measurements and gain greater confidence in your data. The consumables range includes calibrants, sample carrier slides and more.
To streamline your macro mass photometry measurements, use SizeFerence, Refeyn’s custom calibrant for the KaritroMP.

The new KaritroMP is a benchtop instrument that characterizes large viral vectors. Ideal for informing process development in preclinical settings and early drug development, it analyzes samples of adenovirus vectors (AdV) and lentivirus vectors (LVV) in just minutes. The instrument offers essential vector analysis capabilities, including the assessment of sample purity and stability:
Fast, simple, qualitative AdV and LVV analysis
Differentiation of multiple populations, even those of the same size
Evaluation of relative changes across samples, from crude to purified

The MassGlass KV sample carrier is an essential tool for rapid, consistent, and reproducible characterization of adenovirus and lentivirus vectors. Designed for use in a controlled environment, it enables the safe handling of BSL-2 samples.

By using Refeyn consumables, you can spend less time preparing for measurements and gain greater confidence in your data. The consumables range includes calibrants, sample carrier slides and more.
To streamline your macro mass photometry measurements, use SizeFerence, Refeyn’s custom calibrant for the KaritroMP.
What mass photometry users are saying
“The data look great, are easier and quicker to obtain vs other methods (still developing an HPLC method and outsourcing AUC from months). E/F is useful info at R&D stage and academic understanding of what’s going on. “
