Rapid, reliable antibody aggregation analysis with mass photometry
Rapid, reliable aggregation analysis
Antibody aggregation can pose significant challenges in monoclonal antibody manufacturing, affecting both product quality and safety. Access to rapid and convenient analytical methods to detect aggregates during the monoclonal antibody manufacturing process can be extremely valuable.
With Refeyn’s mass photometry solutions, you can compare aggregation levels across different candidates without complex method development, assess antibody aggregation directly from IV bag samples in minutes and derisk by evaluating early using as little as 30 ng of sample.
Mass photometry delivers precise, label-free antibody aggregation analysis without the need for concentration steps or complex preparation, streamlining in-process analysis and accelerating manufacturing workflows. Whether working with monoclonal antibodies, bispecifics, or other modalities, mass photometry offers a faster, simpler way to enhance your antibody production process.
Mass photometry is compatible with most membrane mimetics and offers a non-destructive method for optimizing extraction and purification conditions – enabling structural studies of transmembrane proteins by cryo-EM, X-ray crystallography and other methods.
Benefits of mass photometry for antibody aggregation analysis
1. Direct mAb analysis from IV bag samples
Skip the cumbersome concentration steps typically required for traditional methods, such as size exclusion chromatography with multi-angle light scattering (SEC-MALS). With mass photometry, you can measure antibody aggregation directly from your nM concentration samples in IV bags, providing real-time insights without altering the sample conditions.
2. Ultra-low sample input
Requiring just 30 ng of sample, mass photometry enables you to perform detailed aggregation analysis at nanomolar concentrations. By conserving precious antibody samples, you can extend your analysis to more conditions, accelerating development timelines without compromising accuracy.
3. Results in under 5 minutes
Get fast results with minimal preparation. Mass photometry delivers results in under 5 minutes, allowing you to make quicker, data-driven decisions throughout your manufacturing process. No complex method development, just one universal dilution step, whether you are working with mAbs, bispecifics, or other modalities.
4. No columns, no labels, no hassle
Mass photometry is both column-free and label-free, reducing the risk of interactions or artifacts that can arise from using columns or tags. This maximizes the accuracy of antibody aggregation analysis data, giving you confidence in your analysis across a variety of modalities, including bispecifics and antibody-drug conjugates (ADCs).
5. Accelerate your antibody manufacturing timelines
By enabling rapid screening of up to 24 conditions in just 90 minutes with the TwoMP Auto, mass photometry significantly accelerates antibody manufacturing workflows. Early detection of mAb aggregation issues allows for faster adjustments, reducing development bottlenecks and improving overall process efficiency.
Resolve antibody aggregation and fragmentation
Antibody aggregation and fragmentation can significantly undermine the efficacy and safety of antibody-based products. Being able to detect these issues at various stages of product development is crucial for maintaining high-quality therapeutics. In addition to aggregation and fragmentation events, forced degradation studies are also routinely conducted to evaluate the stability of antibodies, ensuring that they perform as intended even under stress.
Mass photometry presents a sensitive and convenient solution for analyzing these key critical quality attributes of antibodies. It can detect contaminants within antibody samples down to picomolar concentrations, operating effectively across a broad range of buffers and experimental conditions. The technique also integrates seamlessly with other analytical techniques such as SEC-MALS.
Figure 1: Exposure of antibody samples to three different stress conditions (light, pH change, or peroxide). Samples were measured at 10 nM concentration using 5 μl on the TwoMP Auto. Results show that stress conditions 1 and 3 promote aggregation (specifically, the formation of dimers and trimers), relative to the control sample.
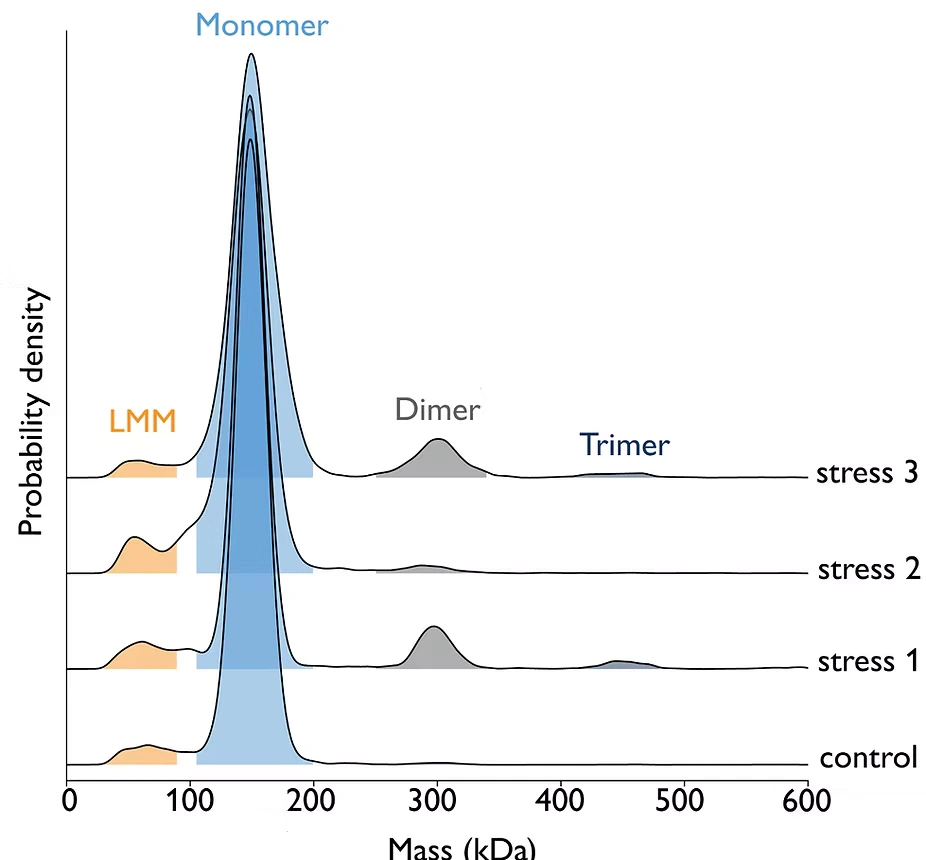
To further improve turnaround times in antibody aggregation analysis, Refeyn provides the Antibody Stability Software Module – which automatically quantifies aggregation in large sample batches in a fraction of the time required for manual analysis, providing statistical analysis for replicates.
The strengths of mass photometry make it a game-changer for fast, in-process analysis of sample purity and stability, transforming the landscape of antibody analytics by providing convenience, ensuring quality and accelerating development.
Mass photometry solutions for antibody aggregation analysis
- TwoMP
-

For molecular mass measurements with unmatched sensitivity, speed and simplicity of use, a TwoMP mass photometer offers a wide mass range and single-molecule resolution.
High-fidelity measurements of molecular mass
Little sample required
Intuitive acquisition and data analysis software
Easy setup – a compact, benchtop instrument with minimal installation requirements
- TwoMP Auto
-

The automated mass photometer frees up operator time and offers enhanced precision, enabling rapid measurement of multiple samples with low sample consumption.
Rapid, automated measurement of multiple samples
Ideal for screening and titration assays
Highly reproducible data with user-friendly software
- Refeyn consumables
-
 By using Refeyn consumables, you can spend less time preparing for measurements and gain greater confidence in your data. The consumables range includes calibrants, sample carrier slides and more.
By using Refeyn consumables, you can spend less time preparing for measurements and gain greater confidence in your data. The consumables range includes calibrants, sample carrier slides and more. - Antibody Stability Module
-
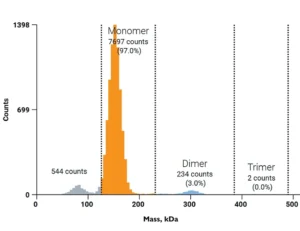 This software module characterizes aggregation in large batches of antibody samples in a fraction of the time required for manual analysis. It supports grouping by replicate and antibody type, and can distinguish and quantify different oligomeric species based on the expected monomer mass.
This software module characterizes aggregation in large batches of antibody samples in a fraction of the time required for manual analysis. It supports grouping by replicate and antibody type, and can distinguish and quantify different oligomeric species based on the expected monomer mass.

For molecular mass measurements with unmatched sensitivity, speed and simplicity of use, a TwoMP mass photometer offers a wide mass range and single-molecule resolution.
High-fidelity measurements of molecular mass
Little sample required
Intuitive acquisition and data analysis software
Easy setup – a compact, benchtop instrument with minimal installation requirements

The automated mass photometer frees up operator time and offers enhanced precision, enabling rapid measurement of multiple samples with low sample consumption.
Rapid, automated measurement of multiple samples
Ideal for screening and titration assays
Highly reproducible data with user-friendly software


Testimonials from mass photometry users
Mass photometry fits perfectly into our analytical workflows when we know there are additional species in our samples [such as aggregates] but we don’t know their mass or their oligomeric state. Now we finally have a suitable technology to fill that gap.”
Dr. Martin E.
experienced scientist at one of the top 5 pharmaceutical companies
“We are very limited in sample volume and concentration in some projects. We have been very surprised with how low you can go in terms of absolute sample concentration.”
Dr. Martin E.
experienced scientist at one of the top 5 pharmaceutical companies
Dr. Martin E.
experienced scientist at one of the top 5 pharmaceutical companies
View further resources related to membrane protein analytics
- Selected publications
-
- Cramer, D. A. T., Franc, V., Heidenreich, A. K., Hook, M., Adibzadeh, M., Reusch, D., Heck, A. J. R., and Haberger, M. (2023). Characterization of high-molecular weight by-products in the production of a trivalent bispecific 2+1 heterodimeric antibody. MAbs, 15(1). [Link]
- This study demonstrates how mass photometry can effectively characterize high-molecular-weight by-products in complex bispecific antibodies, providing key insights into antibody aggregation during production.
- den Boer, M.A., Lai, S.H., Xue, X., van Kampen, M.D., Bleijlevens, B. and Heck, A.J., 2021. Comparative analysis of antibodies and heavily glycosylated macromolecular immune complexes by size-exclusion chromatography multi-angle light scattering, native charge detection mass spectrometry, and mass photometry. Analytical Chemistry, 94(2), pp.892-900. [Link]
- This paper compares mass photometry with native mass spectrometry and SEC-MALS, demonstrating that mass photometry effectively measures antibody aggregation, even in complex samples.
- Parkinson, J., Hard, R. and Wang, W., 2023. The RESP AI model accelerates the identification of tight-binding antibodies. Nature Communications, 14, 454. [Link]
- This study demonstrates how mass photometry provides a reliable method for detecting antibody aggregation, particularly for highly sensitive samples such as scFv antibodies.
- Cramer, D. A. T., Franc, V., Heidenreich, A. K., Hook, M., Adibzadeh, M., Reusch, D., Heck, A. J. R., and Haberger, M. (2023). Characterization of high-molecular weight by-products in the production of a trivalent bispecific 2+1 heterodimeric antibody. MAbs, 15(1). [Link]
- App notes & Tech notes
-
App note: Assessing protein samples by mass photometry and size exclusion chromatography
App note: Characterization of forced antibody degradation with automated mass photometry
Tech note: Streamline antibody stability analysis with mass photometry
- Comparing mass photometry and SEC for antibody aggregation assessment
- Brochures
-
- Webinars
-
- Refeyn posts
-
- Cramer, D. A. T., Franc, V., Heidenreich, A. K., Hook, M., Adibzadeh, M., Reusch, D., Heck, A. J. R., and Haberger, M. (2023). Characterization of high-molecular weight by-products in the production of a trivalent bispecific 2+1 heterodimeric antibody. MAbs, 15(1). [Link]
- This study demonstrates how mass photometry can effectively characterize high-molecular-weight by-products in complex bispecific antibodies, providing key insights into antibody aggregation during production.
- den Boer, M.A., Lai, S.H., Xue, X., van Kampen, M.D., Bleijlevens, B. and Heck, A.J., 2021. Comparative analysis of antibodies and heavily glycosylated macromolecular immune complexes by size-exclusion chromatography multi-angle light scattering, native charge detection mass spectrometry, and mass photometry. Analytical Chemistry, 94(2), pp.892-900. [Link]
- This paper compares mass photometry with native mass spectrometry and SEC-MALS, demonstrating that mass photometry effectively measures antibody aggregation, even in complex samples.
- Parkinson, J., Hard, R. and Wang, W., 2023. The RESP AI model accelerates the identification of tight-binding antibodies. Nature Communications, 14, 454. [Link]
- This study demonstrates how mass photometry provides a reliable method for detecting antibody aggregation, particularly for highly sensitive samples such as scFv antibodies.
App note: Assessing protein samples by mass photometry and size exclusion chromatography
App note: Characterization of forced antibody degradation with automated mass photometry
Tech note: Streamline antibody stability analysis with mass photometry
- Comparing mass photometry and SEC for antibody aggregation assessment
