Adeno‐associated viruses (AAVs) are small, non-pathogenic viruses commonly used as vehicles for gene-therapy delivery. With an increasing number of clinical studies and success stories in the treatment of genetic disorders, AAVs continue to drive advances in the field. To support the development and production of AAV therapies, there is a need for efficient purification techniques. Here, we discuss the use of iodixanol in AAV purification and demonstrate its compatibility with mass photometry – a rapid, easy-to-use technique with proven accuracy for AAV sample analysis.
Iodixanol is a dimeric non-ionic, iodine-containing medium predominantly used as a radiographic contrast agent. In 1999, Zolotukhin et al. first proposed using an iodixanol density gradient (IOD) for rapid, non-toxic purification of AAVs. Since then, IOD has become a staple method for AAV purification in both academic and industrial laboratories (1). AAV purification strategies depend heavily on available methods, intended scale and specific laboratory requirements. Small-scale research and academic laboratories often need a quick, affordable and serotype-agnostic solution, which affinity or ion-exchange chromatography does not always offer. Even within the industry realm, IOD can often deliver a small-scale, quicker one-step solution, with a desired titer and defined full-capsid percentage.
Because iodixanol is formulated as an iso-osmolar intravenous contrast agent, it has a well-established safety profile and is non-toxic to mammalian cells. Traditional CsCl-based purifications, while effective, are time-consuming (often requiring more than three days) and can reduce infectious titers. IOD purification, by comparison, is highly reproducible and consistently yields robust viral preparations (2). However, it has limitations: Scalability is restricted, the open format is prone to contamination and the densest full-capsid fraction may contain up to 20% empty capsids. Supplementary downstream methods often also need to be applied, as iodixanol can interfere with some analytical techniques (3). Residual iodixanol can absorb and scatter light, mimicking DNA or aggregated particles in UV-Vis or light-scattering instruments, necessitating additional corrections (4).
An ideal workflow would combine the quick IOD purification process with analytics that are unaffected by the IOD components, so we explored the compatibility of IOD purification with mass photometry. Iodixanol‑purified samples are highly concentrated and thus require a dilution step prior to mass photometry analysis; this process also lowers the iodixanol concentration to a range low enough that it does not interfere with the mass photometry measurement.
Another important consideration is the amount of sample required for the analysis. Iodixanol‐gradient fractions are very small, so analyzing multiple samples can consume large amounts of precious material when sample-demanding methods are used. Mass photometry, requiring less than 10 µL and being very economical to run, is therefore an ideal complement to IOD purification. Additionally, mass photometry can distinguish empty, partially filled, and full capsids in a single measurement, providing comprehensive insight into all particle populations – which most traditional methods cannot deliver.
A typical iodixanol gradient for AAV purification comprises layers of 15, 25, 40, 60% (w/v) in an ultracentrifuge tube. The lower-density layers remove cellular debris and other light contaminants; the higher-density layers concentrate full AAV capids and act as a cushion for them.
In collaboration with Boehringer Ingelheim (BI), we evaluated the performance of mass photometry on AAVs purified via IOD. Figure 1 shows the iodixanol gradient. Based on our analysis, Fractions 4–8 contained AAV populations. All of the fractions were sampled, diluted to optimal conditions and analyzed on Refeyn’s SamuxMP mass photometer.
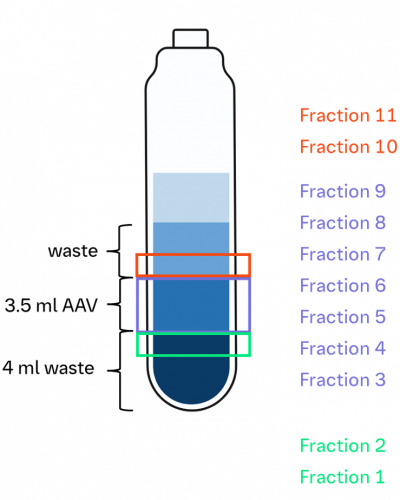
Figure 1. Visual representation of experimental sampling of iodixanol fractions
For the fractions containing AAVs, distinct populations of empty, partially loaded, full and overfull capsids were resolved, and Fraction 5 consistently yielded the highest proportion of full capsids (Figure 2).
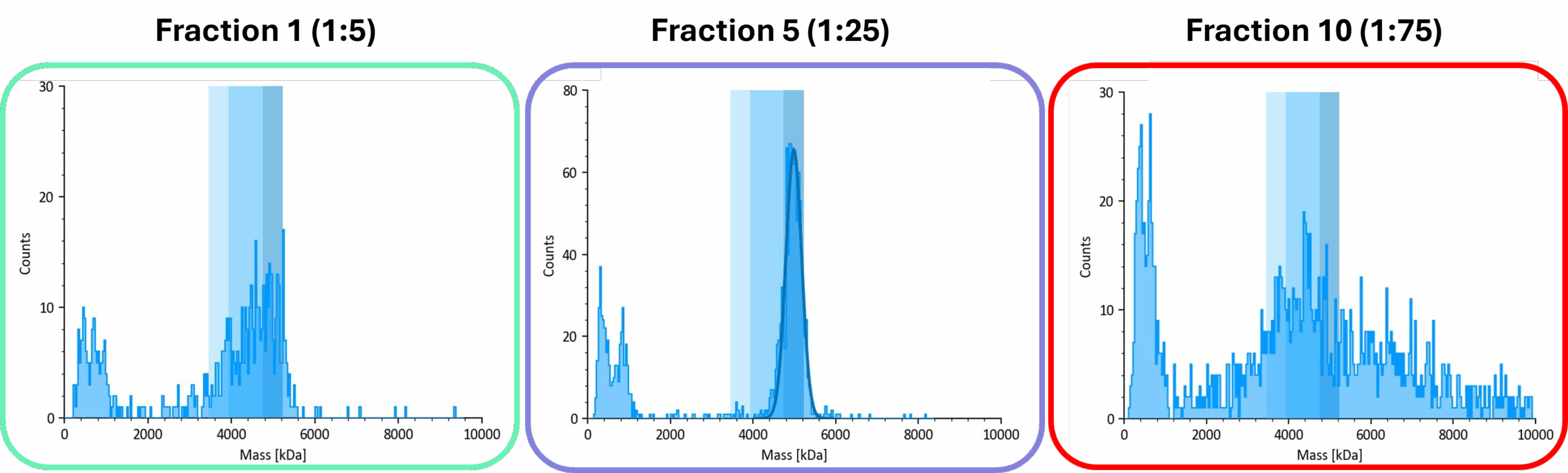
Figure 2. Representative mass photometry data from the three regions of the iodixanol gradient. The frame colors correspond to the gradient regions shown in Figure 1.
Mass photometry determined the percentage of empty, partially loaded and full capsids (Figure 3).
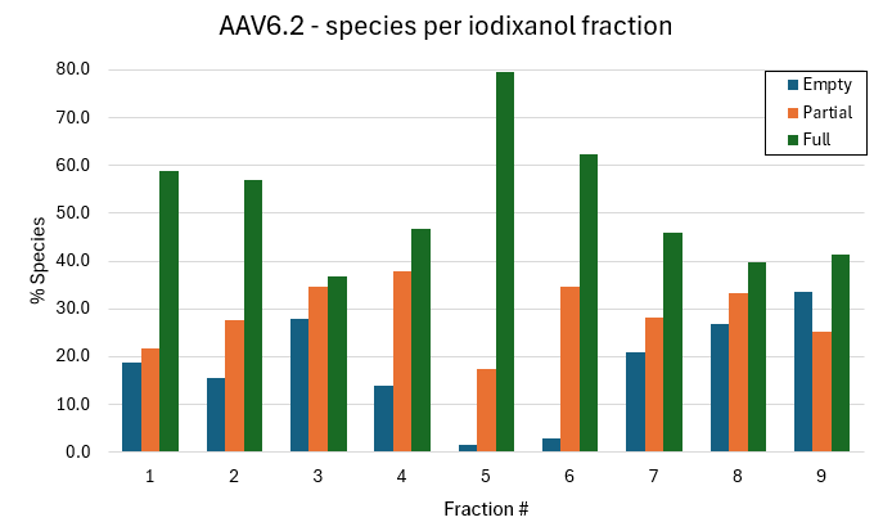
Figure 3. Population analysis of iodixanol fractions of AAV6.2 capsids
Together, these data demonstrate that mass photometry provides fast, accurate assessments of AAV purity and packaging efficiency directly from iodixanol-gradient preparations. Incorporating mass photometry into a small-scale IOD process therefore offers researchers a streamlined workflow from purification to characterization.
Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999
Strobel B, Miller FD, Rist W, Lamla T. Comparative Analysis of Cesium Chloride- and Iodixanol-Based Purification of Recombinant Adeno-Associated Viral Vectors for Preclinical Applications. Hum Gene Ther Methods. 2015
Lam AK, Mulcrone PL, Frabutt D, Zhang J, Chrzanowski M, Arisa S, Munoz M, Li X, Biswas M, Markusic D, Herzog RW, Xiao W. Comprehensive Comparison of AAV Purification Methods: Iodixanol Gradient Centrifugation vs. Immuno-Affinity Chromatography. Adv Cell Gene Ther. 2023
Yang QE, Walton RW, Kudrolli T, Denys N, Lance L, Chang A, Rapid Quality Control Assessment of Adeno-Associated Virus Vectors Via Stunner, GEN Biotechnology 2022