This post was first published in March 2023
Updated on 20 February 2026

Adeno-associated virus (AAV) sample characterization is critical for research, development, and manufacturing processes of gene therapies that involve these viral vectors (Fig. 1). At Refeyn, we have developed the Samux™ line of mass photometry products specifically for AAV analysis, featuring the SamuxMP and SamuxMP Auto mass photometers as well as dedicated consumables.
Here, we discuss the pros and cons of mass photometry when compared to other frequently used methods for AAV analytics, including electron microscopy (EM), analytical ultracentrifugation (AUC), charge detection mass spectrometry (CDMS), quantitative polymerase chain reaction (qPCR) combined with enzyme-linked immunosorbent assay (ELISA), and size exclusion chromatography combined with multi-angle light scattering (SEC-MALS). We aim to provide a straightforward overview of the key analytical techniques used in the AAV space, so you can understand how mass photometry would fit into your AAV analytics toolbox.
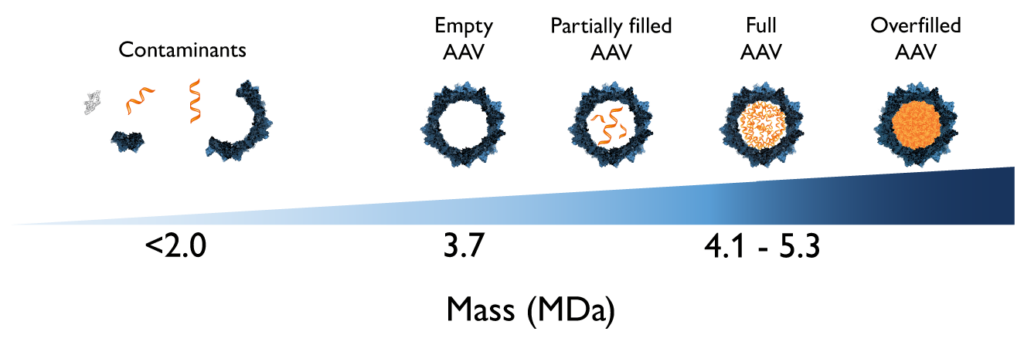
Figure 1. AAV samples can contain a mixture of different species. The main impurities in AAV samples are capsids with no genome content (empty) or that contain an incomplete copy of the recombinant DNA (partially filled). Other impurities like capsid fragments, free nucleic acids, and protein contaminants can be detected in the lower mass ranges. Finally, AAV samples may also contain higher-mass, overfilled capsids, which are still being investigated and are generally treated as impurities unless explicitly defined as part of the product.
Sample characterization can become a bottleneck in AAV development processes. As a result, one practical consideration when choosing between AAV analysis methods is the time each measurement takes.
Techniques like CDMS, EM, and AUC deliver high-quality data, but their long turnaround times can slow development and hold up manufacturing decisions [1]–[4]. Others, like qPCR/ELISA or SEC-MALS, are significantly faster and are frequently used when time is a factor. Mass photometry is currently the fastest available technique for AAV analysis. Individual mass photometry measurements take less than five minutes, and the automated mass photometer SamuxMP Auto runs 24 samples in as little as 90 minutes without user intervention.
Mass photometry’s speed makes it practical not only for characterization, but also for at-line decision-making during process development and manufacturing, enabling rapid screening of conditions, timely fraction selection in downstream purification, faster troubleshooting, and tighter process control without adding delays to the workflow (Fig. 2).
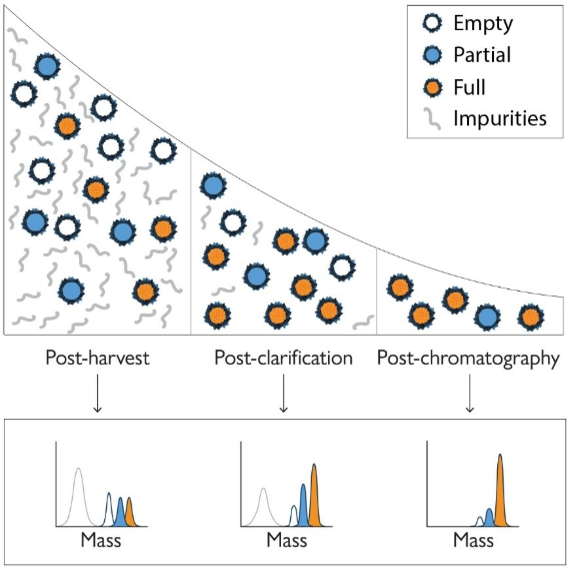
Figure 2. Mass photometry provides valuable information throughout downstream AAV purification processes. At multiple purification stages, mass photometry can be used to quantify empty, partially filled, full, and overfilled capsids, as well as other impurities, enabling rapid, at-line checks across key unit operations during development and manufacturing.
Equipment, running costs, and expertise requirements are all important to consider when evaluating the suitability of an AAV analytical method for a given environment. Among frequently used techniques, both AUC and EM require specialized equipment and highly trained operators to acquire and analyze data. As a result, these techniques are often outsourced and/or reserved for specific characterization studies and later-stage release assays.[1]–[5].
Other AAV characterization approaches, such as qPCR-ELISA, SEC-MALS, and CDMS, can be run on benchtop instruments, which makes them easier to implement [1]–[4], [6], although CDMS requires an advanced native mass spectrometer and considerable expertise for instrument calibration, experimental set-up and data analysis.
Mass photometers fit on a benchtop, require as little as one day of training to operate (Fig. 3), and have running costs of less than $5 per measurement. This makes mass photometry easy to implement in-house for readily available AAV analytics.

Figure 3. Loading a sample onto a mass photometer. Only basic lab skills are required to use mass photometry. An operator simply pipettes the sample onto a sample carrier slide.
If your AAV development and manufacturing processes regularly include multiple capsid serotypes, it is necessary to consider whether that could affect your analytical workflow protocols. AAV characterization methods that depend on high molecular specificity, such as qPCR-ELISA or affinity chromatography, must be optimized for each serotype.
By contrast, methods based on intrinsic physical properties such as hydrodynamic size or particle mass tend to transfer more easily across AAV serotypes and typically require only modest method tuning compared to the methods mentioned above. These approaches include SEC-MALS, AUC, EM, CDMS, and mass photometry. Among them, mass photometry generally requires the least re-optimization across serotypes (Fig. 4)
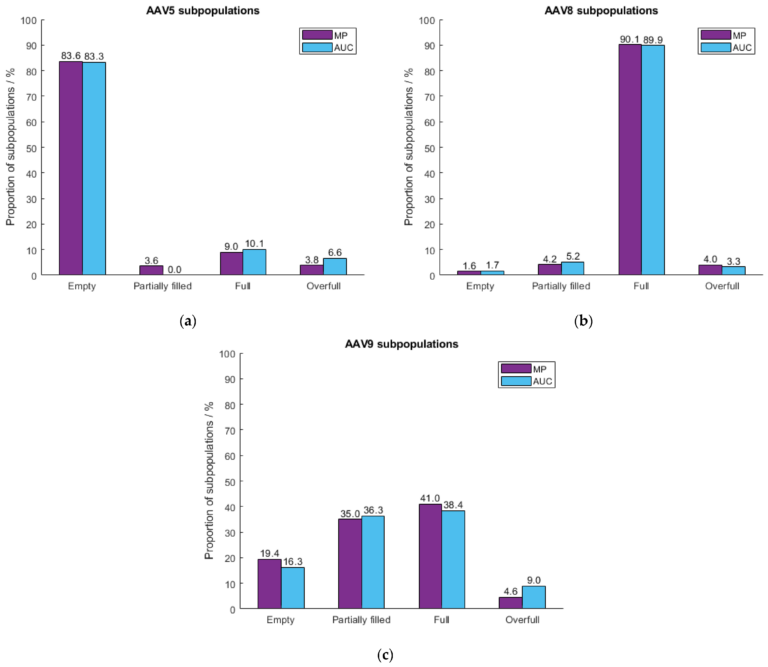
Figure 4. Mass photometry accurately measures AAVs of different serotypes. These data from Wagner et al. (2023) [7] show how mass photometry and AUC output comparable results when quantifying empty, full, partially filled, and overfilled capsids of three different serotypes: AAV5 (a), AAV8 (b), and AAV9 (c). Measurements were taken on SamuxMP.
Monitoring vector production, quality, and process performance during cell culture and harvest enables earlier decisions that reduce risk to development and production. Some analytical techniques, like ELISA/qPCR, can work on crude samples with minimal preparation. Others, like CDMS, EM, SEC-MALS, and AUC, require some sample processing before they can be applied. Mass photometry can also be used to get critical insights into crude AAV samples, with the added advantage that MP is compatible with quick cleanup protocols that take only 1 to 3 hours (in comparison to 24 hours for AUC).
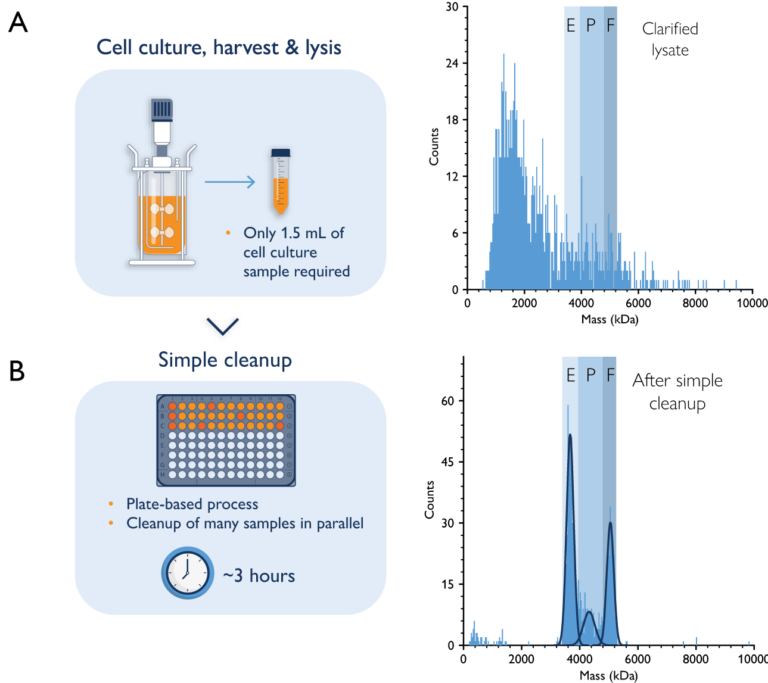
Figure 5. Mass photometry can be used for upstream AAV characterization. Mass photometry analysis of a crude AAV sample during upstream processing (A) and after a simple cleanup step (B). The histograms show the mass ranges of empty (E), partially filled (P), and full (F) capsids. AAV capsids could not be resolved in the crude sample but could be quantified after the cleanup step. These data were collected by Généthon with SamuxMP.
In addition to empty and full AAVs, preparations may include capsids that contain genetic contaminants, such as truncated copies of the intended cargo or fragments of host cell DNA. These fragments are often smaller than the intended cargo, so the AAV capsids containing them are only partially filled in comparison to the ‘full’ capsids in the sample.
Capsids may also be overfilled – containing more cargo than is intended. To determine whether a sample contains partially filled or overfilled AAV capsids, it is important to choose a technique with enough resolution to differentiate capsids that differ in mass. Bulk analysis techniques such as qPCR-ELISA and SEC-MALS do not have this capability, as they measure average properties across the whole sample. Electron microscopy techniques, on the other hand, struggle to resolve the small electron density differences needed to identify heterogeneously loaded capsids [1], [3], [4], [6].
In contrast, AUC, CDMS, and mass photometry (Figs. 4 and 5) characterize the different species in a sample individually at high resolution, enabling detection of populations of empty, partially filled, full, and overfilled capsids [1]–[6]. In terms of accuracy, both AUC and CDMS are considered to have slightly higher mass resolution and lower variability than MP analysis, but results are seen as broadly comparable between the techniques, with MP having a practical advantage due to its fast measurements and ease of use [5], [7], [8].
For processes involved in drug manufacturing, regulatory agencies establish a series of regulations known as good manufacturing practices (GMP), such as Title 21 CFR 11 by the US Food and Drug Administration (FDA) or GMP Annex 11 by the European Union. GMP compatibility is an important consideration when choosing your favorite AAV analysis technique, as it will continue to support your processes once you reach the manufacturing stage. Mass photometry, SEC-MALS, and qPCR/ELISA are all techniques with full GMP support from their manufacturers. EM and AUC are compatible with GMP, but their instrument manufacturers do not provide GMP-compliant software. Finally, CDMS is, for now, not suitable for use in GMP-regulated environments.
Table 1. A summary of the advantages and disadvantages of different methodologies for AAV analytics.
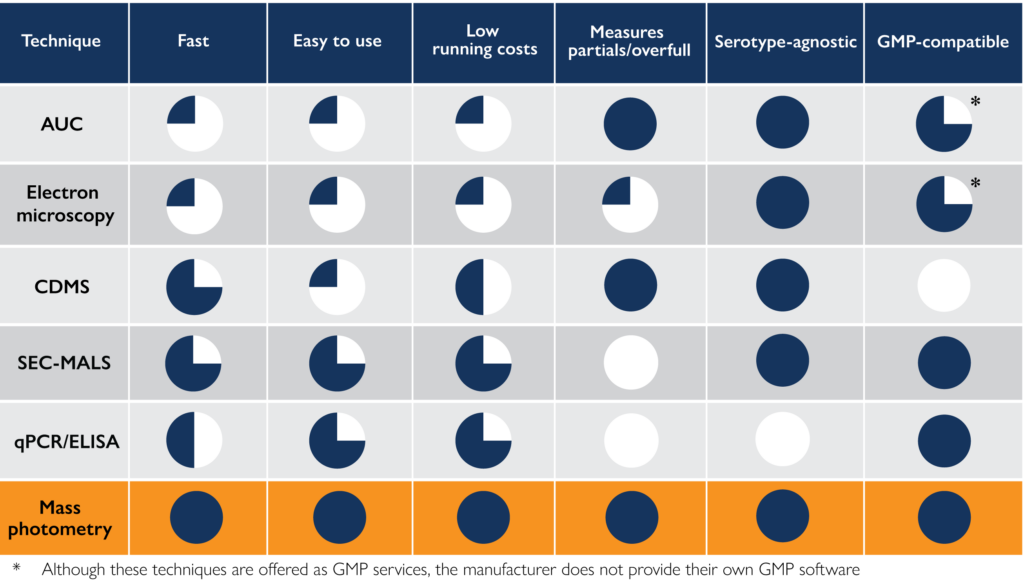
To optimize your AAV development and production processes, it is important to choose AAV characterization technologies that streamline your analytical workflows. Factors to consider when choosing an approach to analyze AAV samples include measurement time, cost, ease of use, and what information it can provide about the capsid populations in the sample.
In this article, we have given an overview of the main techniques available for AAV characterization, comparing them based on their speed, ease of use, and analytical capabilities (see summary in Table 1).
Overall, mass photometry compares favorably to other methods. The information provided by mass photometry is comparable to that of powerful techniques like AUC or CDMS, but with lower time and expertise requirements, lower sample consumption and running costs, and a small instrument footprint. This makes mass photometry a powerful and versatile tool for AAV analytics that can be applied throughout development and manufacturing.
To ask questions, get in touch with our mass photometry experts.
USP AAV standards to support quality testing and characterization
In this webinar, Dr. Anthony Blaszczyk (USP) discusses the first AAV reference materials released by the United States Pharmacopeia. In addition, Drs. Paul Getty and Lauren Tomlinson (Pharmaron) talk about Pharmaron’s strategy to use mass photometry for AAV process development and manufacturing.
Measuring AAV genome length with mass photometry
This application note – created in collaboration with AskBio – shows how mass photometry can be used to measure the encapsidated AAV genome length. The app note showcases how the SamuxMP and SamuxMP auto mass photometers easily detect and quantify empty and full AAV populations. You will also see example data from AAV samples of different serotypes showing expected vs measured AAV genome length, highlighting the precision of mass photometry.
Mass photometry in contract research and manufacturing
Refeyn’s mass photometry technology is trusted by research institutions and pharma organizations worldwide, including many CROs and CDMOs. Find out how its suitability for GMP environments and growing acceptance by regulatory authorities make it useful for teams working in process development or manufacturing.
Recent advances in purification techniques have enhanced AAV sample compatibility with mass photometry in upstream development. Watch this webinar to learn how to overcome the limitations of traditional characterization methods (e.g., AUC, SEC-MALS, TEM) and how mass photometry enhances GMP-regulated environments and process optimization.
[1] M. Penaud-Budloo, A. François, N. Clément, and E. Ayuso, ‘Pharmacology of Recombinant Adeno-associated Virus Production’, Molecular Therapy – Methods & Clinical Development, vol. 8, pp. 166–180, Mar. 2018, doi: 10.1016/j.omtm.2018.01.002.
[2] J. C. Grieger, V. W. Choi, and R. J. Samulski, ‘Production and characterization of adeno-associated viral vectors’, Nature Protocols, vol. 1, no. 3, Art. no. 3, Aug. 2006, doi: 10.1038/nprot.2006.207.
[3] A. L. Gimpel et al., ‘Analytical methods for process and product characterization of recombinant adeno-associated virus-based gene therapies’, Molecular Therapy – Methods & Clinical Development, vol. 20, pp. 740–754, Mar. 2021, doi: 10.1016/j.omtm.2021.02.010.
[4] A. K. Werle et al., ‘Comparison of analytical techniques to quantitate the capsid content of adeno-associated viral vectors’, Molecular Therapy – Methods & Clinical Development, vol. 23, pp. 254–262, Dec. 2021, doi: 10.1016/j.omtm.2021.08.009.
[5] E. H. T. M. Ebberink, A. Ruisinger, M. Nuebel, M. Thomann, and A. J. R. Heck, ‘Assessing production variability in empty and filled adeno-associated viruses by single molecule mass analyses’, Molecular Therapy – Methods & Clinical Development, vol. 27, pp. 491–501, Dec. 2022, doi: 10.1016/j.omtm.2022.11.003.
[6] E. A. Green and K. H. Lee, ‘Analytical methods to characterize recombinant adeno-associated virus vectors and the benefit of standardization and reference materials’, Current Opinion in Biotechnology, vol. 71, pp. 65–76, Oct. 2021, doi: 10.1016/j.copbio.2021.06.025.
[7] Wagner C, Fuchsberger FF, Innthaler B, Lemmerer M, Birner-Gruenberger R. ‘Quantification of empty, partially filled and full adeno-associated virus vectors using mass photometry’. International journal of molecular sciences. Jul. 2023, 3;24(13):11033. https://doi.org/10.3390/ijms241311033.
[8] Townsend JA, Li S, Sweezy L, Liu N, Rosconi MP, Pyles EA, Zhi L, Liu D, Wu Z, Qiu H, Shameem M. Comparative analysis of empty and full adeno-associated viruses under stress conditions by anion-exchange chromatography, analytical ultracentrifugation, and mass photometry. Journal of Pharmaceutical Sciences. Feb. 2025;114(2):1237-44. https://doi.org/10.1016/j.xphs.2025.01.005.