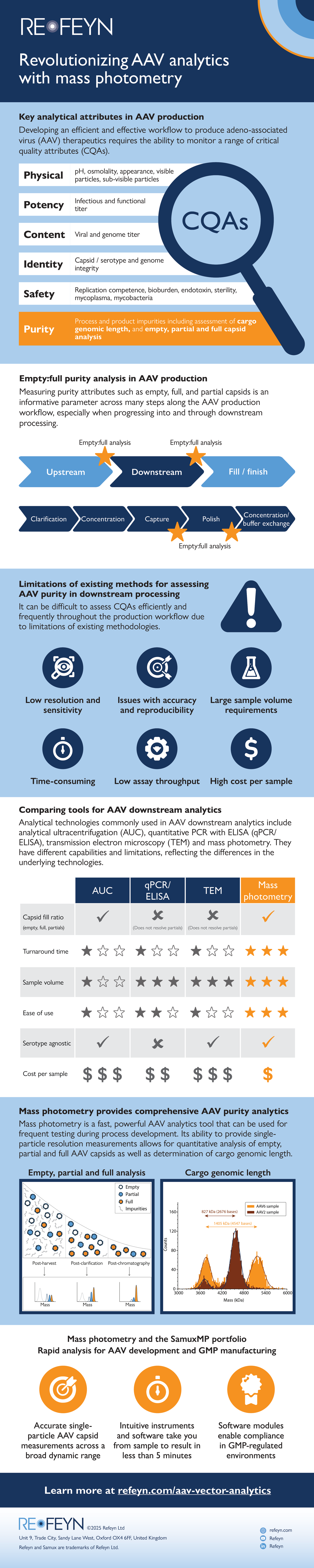
Mass photometry measures the mass of single particles in solution without disruptive preparation or labels. This powerful simplicity makes it a versatile technique, with a role to play in a variety of applications, from assessing antibody aggregation to measuring RNA length. Among these, characterizing AAVs is one where mass photometry really shines and is quickly being adopted across pharmaceutical companies and CROs.
Developing an AAV production pipeline and scaling it up to manufacture safe and effective pharmaceuticals is far from straightforward. Across AAV development and production, critical quality attributes (CQAs) of the samples need to be accurately assessed without bogging down the process. Let’s take a detailed look at these CQAs, the current analytical challenges faced by AAV development and production, and how mass photometry addresses them with efficient, in-line AAV sample characterization.
Application details on Refeyn’s website: Rapid analytics of AAV vectors with mass photometry – Refeyn
Application notes on AAV characterization: Application Notes – Refeyn
YouTube tutorial: Ready, Set, Quantify: How to Analyze Empty, Full, and Partial AAVs in less than 5 minutes
Webinar: Advancing AAV Therapeutics with Mass Photometry: SamuxMP in Action
Highlighted scientific publications: