Streamlining AAV analytics in gene therapy manufacturing workflows
Application of mass photometry in GMP-regulated environments for characterization of AAV samples

Read this whitepaper to learn how the Samux software package for GMP-regulated environments complies with GMP manufacturing regulations. Find out the main components of the GMP software package and their features. See detailed information on how the Samux software package for GMP provides user management and access, audit trails, electronic signatures and individually traces every dataset. Finally, find data showcasing the automated workflow that detects empty, full and partially filled AAV capsids in a sample based on basic user input.
Download the whitepaper
Further Reading on AAV Analytics
AAV analytics in GMP-regulated environments
AAV manufacturing environments are GMP-regulated and subject to a series of rules established by agencies such as the US Food and Drug administration (FDA) and the European Medicines Agency (EMA). These regulations require that any software used in AAV manufacturing workflows have features that ensure data traceability and reproducibility. In addition, instruments to be used within AAV manufacturing processes need to be qualified at different stages of production and implementation. These qualifications include design, installation and operational qualifications (DQ, IQ, OQ).
New AAV analytical technology now available for use in AAV manufacturing workflows
SamuxMP, the mass photometer tailored for AAV analytics, can be used in GMP-regulated environments as it has a compliant software that fulfills the FDA and EU requirements for AAV manufacturing including:
User management and access control, including several privilege levels
Full data traceability, unique ID assigned for each raw dataset
Detailed audit trail and electronic signatures for full accountability
The Refeyn service teams also provide installation and operational qualifications for instruments destined to AAV manufacturing facilities, including documentation and training upon request
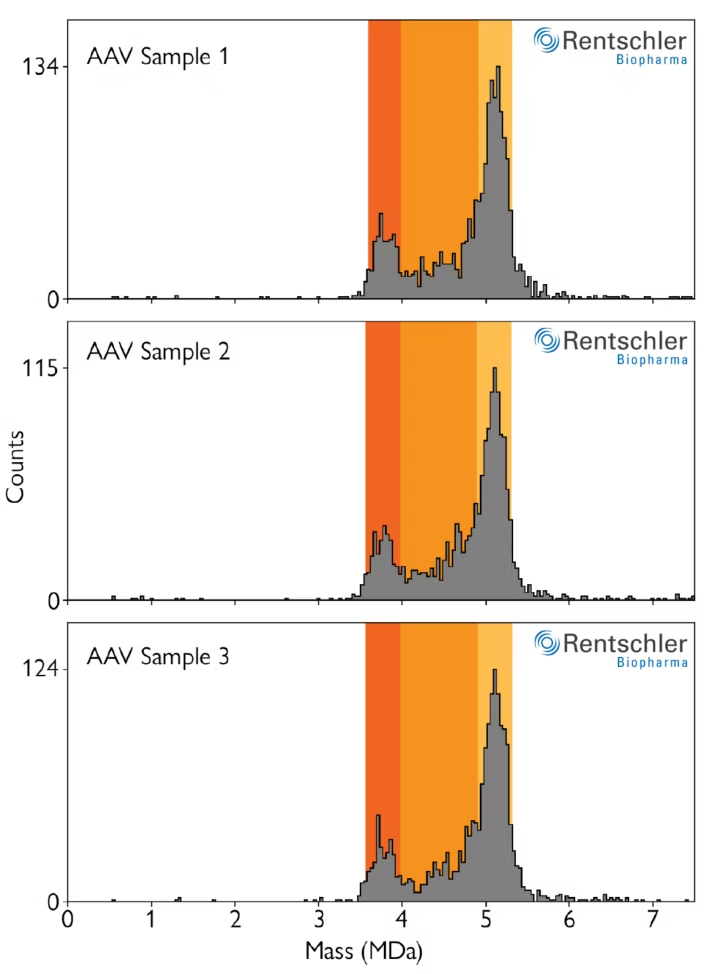
Streamlined, reproducible workflows for AAV characterization in GMP-regulated environments
Refeyn’s software package for AAV manufacturing environments includes automated workflows to streamline data analysis, as well as standardize it between operators. In the automated mode, mass ranges are automatically defined to classify detected AAVs into empty, full and partially filled populations.
These ranges are automatically defined based on user inputs for the expected mass of the empty capsids and the size of the genomic payload. Manual and semiautomated modes are also available for users with advanced permissions.
Image: Three measurements of an AAV sample using an automated workflow that detects empty, full and partially filled AAV capsids
