The PROTAC technology
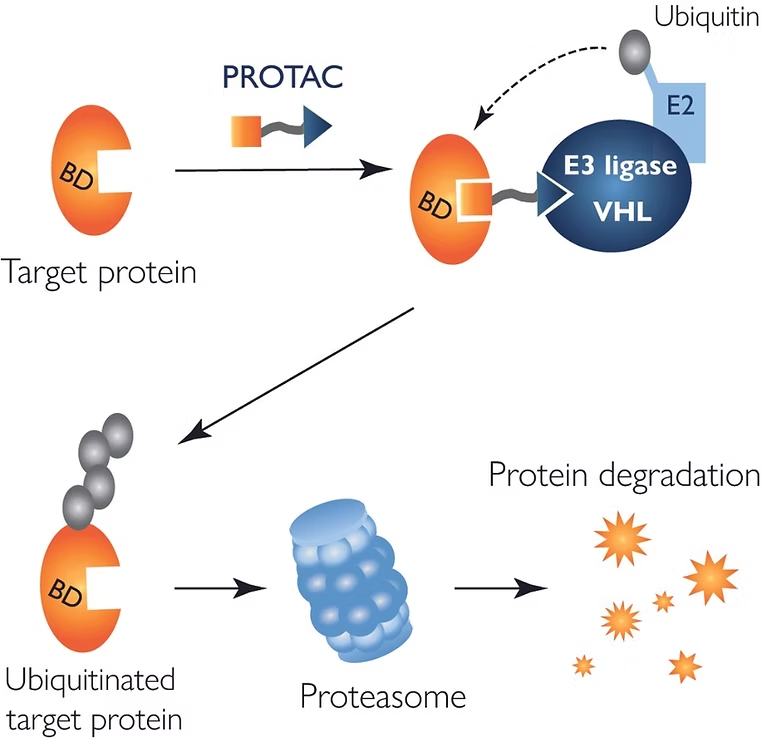
How to measure PROTAC ternary complex formation?
When applied to PROTAC assays, mass photometry – a label-free technology – offers insights into the dynamics of ternary complex formation. With the ability to measure the mass of single biomolecules in solution, it enables the characterization of PROTAC-driven interactions with the target protein and the E3 ligase. This means that you can use mass photometry to assess a range of behaviors that are important for the PROTAC mechanistic function: ternary complex formation, cooperativity, stoichiometry and ‘hook effect’.
Biophysical characterization of the PROTAC ‘hook effect’
Mathematical models predict a bell-shaped dependency on PROTAC concentration. At high concentrations, ineffective binary complexes are observed, which compete with effective ternary complexes. Competition leads to a decline in the formation of ternary complexes, ultimately impacting the potency of the PROTACs. This is known as the ‘hook effect’. Mass photometry can be used to quantify relative concentrations of intermediate species and assess their cooperativity effects. More specifically, you can determine the concentration range where maximal complex formation occurs and identify PROTAC compounds with significant positive cooperativity in the ternary complex, which do not present an observable ‘hook effect’.
To learn how mass photometry can help characterize protein interactions and complexes
Mass photometry for the study of PROTAC ternary complexes
Additional resources
BLOG: Exploring the structural dynamics of shelterin with mass photometry
Learn how mass photometry can be used to study the compositional heterogeneity, stoichiometry, and stability of a multimeric complex. Find out how it complements other bioanalytical techniques, such as native mass spectrometry and negative-stain EM.

WEBINAR: The evolution of carbon fixation: Studying Form I Rubisco using ancestral sequence reconstruction and mass photometry
Discover how mass photometry can be used in combination with ancestral sequence reconstruction to determine when an ancestral enzyme gained its structural complexity and how it started to interact with a novel subunit.
To discuss how mass photometry can be applied to the characterization of PROTAC complex formation
More Application Notes
Browse through our catalogue of application notes highlighting some recent case studies featuring mass photometry.
