Characterizing antibody aggregation with mass photometry
Importance of assessing antibody aggregation in biopharmaceutical products
Antibody aggregation is a critical concern in the development of biopharmaceuticals, including therapeutic antibodies such as monoclonal antibodies, bispecific antibodies, and antibody-drug conjugates.
Antibody aggregation poses significant challenges because it can negatively impact the efficacy, safety, and stability of these biopharmaceutical products. Aggregated antibodies may lose their bioactivity, trigger unwanted immunogenic responses in patients, and shorten the shelf life of the product. Therefore, in biopharmaceutical development, minimizing the propensity of antibodies to form aggregates during manufacturing and storage is essential for product quality and patient safety.
How can mass photometry characterize antibody stability?
Mass photometry is a cutting-edge analytical technique that can be used for the characterization of antibody aggregation and degradation, in solution. This label-free bioanalytical tool provides rapid insights into changes in the behavior of molecules, making it well-suited for monitoring the stability of biopharmaceuticals such as therapeutic antibodies.
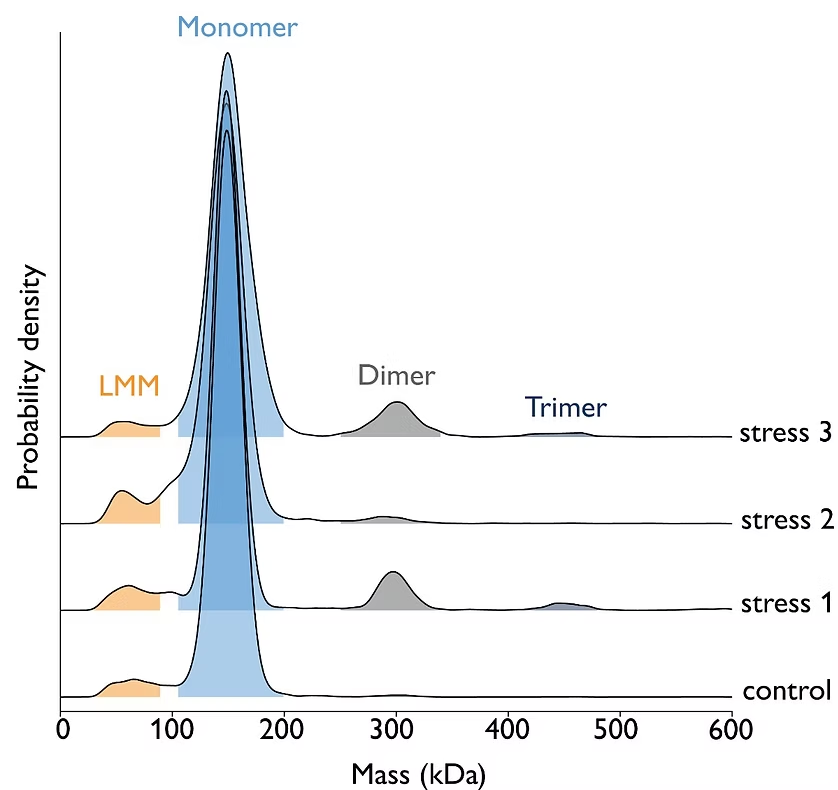
Mass photometry can be employed to study the response of antibodies to various stressors, such as light exposure, pH fluctuations, and exposure to peroxide. In a forced degradation study, mass photometry helped detect and quantify fragmentation and aggregation of antibody molecules.
Image: Mass photometry can assess antibody stability in response to different stressors. For experimental details, download the app note.
Advantages of mass photometry for the study of mAb aggregation
Mass photometry offers several advantages for studying antibody aggregation. Unlike traditional methods like size-exclusion chromatography coupled with multi-angle light scattering (SEC-MALS), mass photometry is a column-free approach, reducing the complexity of sample optimization. It is compatible with a wide range of buffers, minimizing the need for buffer adjustments.
Mass photometry ability to work with small sample quantities streamlines the experimental process and minimizes material consumption. Moreover, the user-friendly operation and intuitive data analysis provided by mass photometry enable rapid data-driven decision-making during biopharmaceutical development, supporting process optimization and developability assessment. As a result, mass photometry serves as a valuable tool for comprehensive biopharmaceutical product characterization, including monoclonal and bispecific antibodies, facilitating the identification and mitigation of aggregation issues in drug development.
To learn how mass photometry can help measure antibody aggregation in solution
Mass photometry in a forced antibody degradation study
Learn how mass photometry can help assess changes in antibody behavior under various stress conditions. Discover how it can be used to analyze the fragmentation and aggregation of therapeutic antibodies in response to stressors like light, pH variations, and peroxide exposure. Find out the advantages automated mass photometry can offer, in terms of processing multiple samples with high reproducibility, across a wide mass range. Gain a deeper understanding of how mass photometry can support biopharmaceutical development and help ensure the quality and safety of monoclonal and bispecific antibodies.
Additional resources
BLOG: Overcoming analytical bottlenecks with mass photometry – with structural biologists from big pharma
Learn how mass photometry was used for the characterization of antibody and gene therapy samples at one of the top 5 pharmaceutical companies. Discover how the TwoMP Auto mass photometer helped biopharma experts overcome their experimental bottlenecks and how it compares with other bioanalytical methods.


WEBINAR: Monitoring aggregation levels of biosimilar mAbs using mass photometry and SEC
Learn how mass photometry compares with size-exclusion chromatography in measuring aggregation levels of monoclonal antibodies. Discover the pros and cons of each technology and the factors that should be considered when analyzing and comparing data generated by these different techniques.
To discuss how mass photometry can be applied in forced degradation antibody studies
More Application Notes
Browse through our catalogue of application notes highlighting some recent case studies featuring mass photometry.
