Explore how mass photometry compares to traditional pre-screening tools—and how recent studies reveal its growing role and applications in pre-cryo-EM workflows.
The impact of cryo-electron microscopy (cryo-EM) on structural biology has been transformational. Recognized with the Nobel Prize in Chemistry in 2017, cryo-EM has opened the door to atomic-level insights into some of the most complex biomolecular assemblies. Yet, despite its power, the success of any cryo-EM experiment still rests heavily on one critical factor: The quality of the starting sample.
Sample heterogeneity, aggregation and dynamic oligomeric states can all compromise cryo-EM outcomes. Without proper sample characterization, these issues can go unnoticed—only to surface during vitrification, where they can impact ice quality, particle distribution and grid consistency. The result? Low-quality micrographs, datasets that are too noisy or inconsistent for high-resolution reconstruction and precious EM instrument time squandered.
Since so much hinges on sample quality, effective pre-screening is essential. However, conventional tools—such as dynamic light scattering (DLS), size-exclusion chromatography (SEC), or negative stain electron microscopy (ns-EM)—sometimes fall short. These techniques can be expensive, time-consuming, sample-demanding, or may lack the resolution needed to make confident decisions before committing to grid freezing (Figure 1).
Figure 1. Mass photometry offers single-particle resolution. For cryo-EM sample optimization and screening, single-particle measurement provides the resolution needed for visibility of small population differences to ensure monodispersity and sample homogeneity. Single-particle measurement tools include negative stain electron microscopy (nsEM) and mass photometry, and provide information related to individual species in a sample.
Mass photometry offers a smarter solution. A convenient, label-free, low-volume approach, mass photometry delivers rapid, quantitative insights into sample composition, revealing sample heterogeneity and oligomeric distributions within minutes. Increasingly adopted by leading cryo-EM centers—including national NIH-funded cryo-EM centers in the United States—mass photometry is increasingly being employed to help researchers de-risk their workflows and maximize their cryo-EM outcomes.
Before committing your sample to grid freezing, it’s worthwhile to first determine exactly what’s in the tube. Mass photometry offers a fast, intuitive way to deliver real-time insights into sample composition with minimal hands-on time and virtually no sample preparation. With no labelling, staining or specialist handling required, mass photometry offers a “plug-and-play” workflow that enables users to quickly learn how to run measurements and interpret their data with confidence. A recent review of cryo-EM advances described mass photometry as “a promising technology for initial sample characterization by providing useful preliminary information about a sample rapidly and at low cost.” (Chua et al., 2022).
Another recent commentary highlights the value of mass photometry for pre-screening as well as further investigation of a structural model, saying that “the speed, cost, and universal applicability of [mass photometry] make it a powerful protein characterization method prior to or following structural studies” (Vostal, 2025). This article compares mass photometry to other techniques, noting that its advantages include that analysis is carried out in native conditions, and its speed and ease of use – in addition to favorable accuracy, resolution and dynamic range.
Mass photometry works by detecting the light scattered by individual molecules as they land on a glass slide. By analyzing the resulting interference patterns, it determines the mass of each particle with high precision—all in solution and under native-like conditions. Within minutes, mass photometry can reveal whether a sample contains the expected species, whether it’s monodisperse or aggregated, and whether it exists in a desired oligomeric state.
In pre-cryo-EM screening, a favorable, homogeneous sample typically returns a single sharp peak corresponding to the expected molecular mass. In contrast, additional peaks or broader distributions may indicate sample heterogeneity, aggregation or degradation—issues that could compromise vitrification or significantly increase the time required to image enough particles for 3D reconstruction.
As an example, the illustration in Figure 2 shows a hypothetical protein assembly that could disassemble or form aggregates, along with data from two samples. The first contains mainly the intact assembly, while the second consists mainly of disassembled components. This type of information is invaluable when selecting which sample to use for further study.
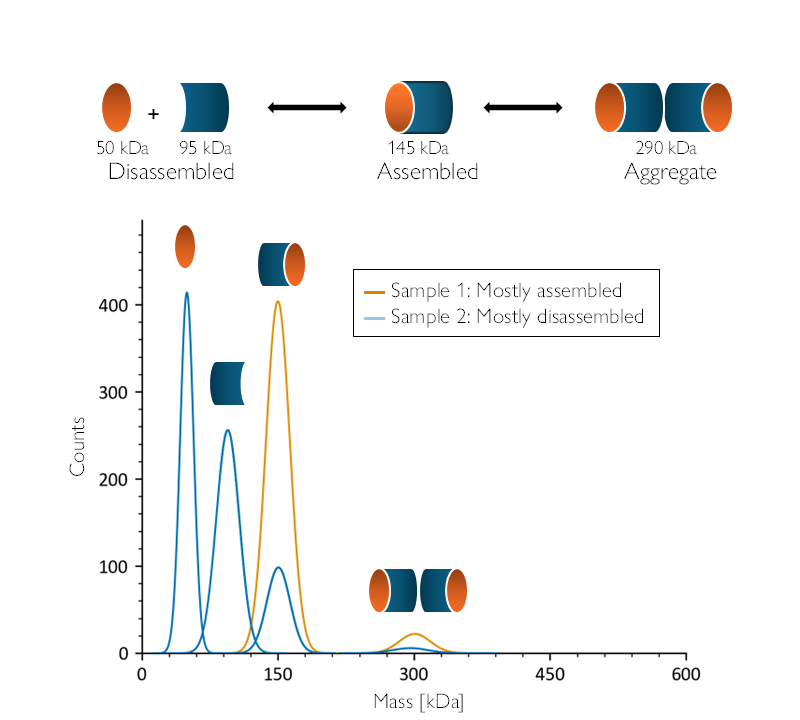
Figure 2: Mass photometry provides vital information for sample screening and selection. A theorized protein assembly (top) could disassemble or form aggregates. Mass photometry (bottom) would clearly differentiate these two hypothetical samples, showing that Sample 1 (orange) contains mainly complete assemblies—as evidenced by the single large peak at ~150 kDa. Meanwhile, Sample 2 (dark blue) is mainly disassembled—as evidenced by the presence of three significant peaks around ~50, ~100 and ~150 kDa.
For years, researchers have relied on tools like ns-EM, DLS, and SEC-MALS to screen samples before vitrification—each bringing its own set of limitations in speed, resolution and resource demands. We next explore the key differences between mass photometry and other tools used to screen samples prior to cryo-EM analysis.
Negative stain electron microscopy (ns-EM)
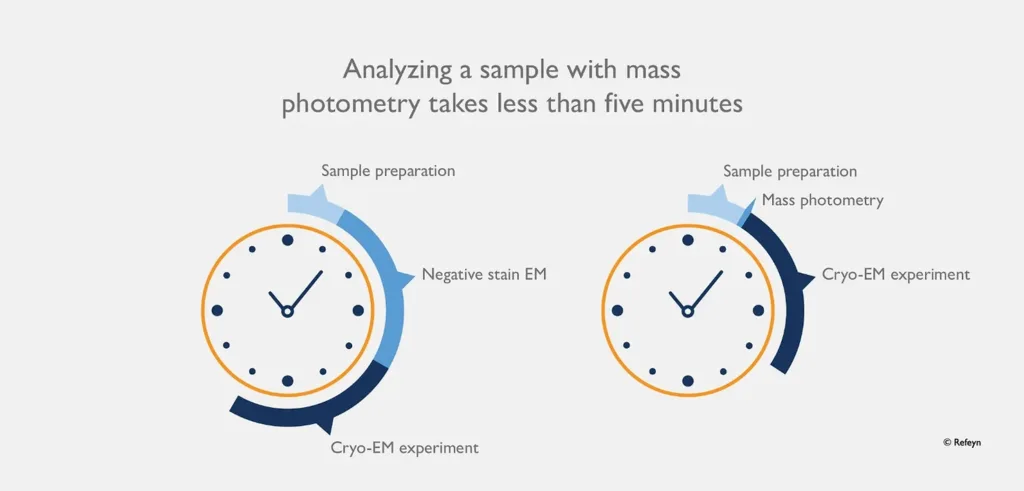
Figure 3: Saving time. Mass photometry dramatically improves the efficiency of characterizing samples prior to cryo-EM.
Dynamic light scattering (DLS)
Size-exclusion chromatography/multi-angle light scattering (SEC/SEC-MALS)
Integrating mass photometry into cryo-EM workflows, with its ease-of-use and minimal sample preparation, enables users to confidently determine sample quality, dramatically reducing wasted effort and resources. This is well illustrated by a 2022 study (Niebling et al., 2022), which outlined the development of a systematic screening pipeline for cryo-EM grid preparation. The researchers combined several biophysical tools—including DLS and mass photometry—to assess sample quality before vitrification.
In another comparative study (Sonn-Segev et al., 2020), researchers demonstrated that mass photometry could detect and quantify assembly states with greater speed than ns-EM but equivalent accuracy (Figure 4). Mass photometry provided quantitative mass distributions to inform sample selection and reduce the risk of downstream failure.
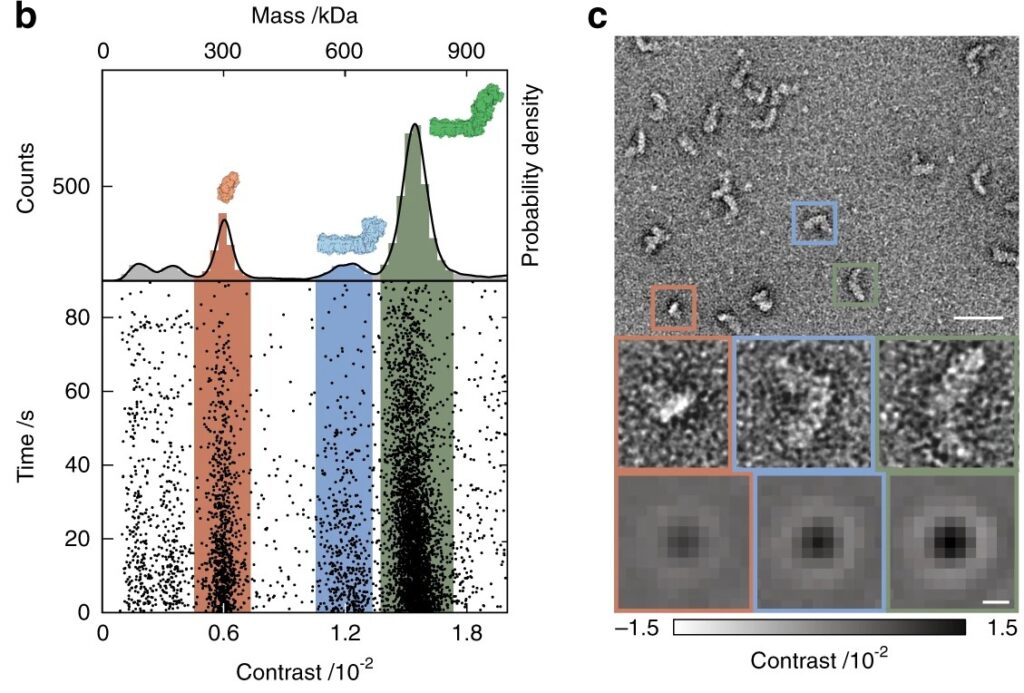
Figure 4: Mass photometry agrees with ns-EM. Mass photometry (left) and ns-EM (right) analyses of the respiratory complex I proton pump from E. coli, a multi-subunit membrane-bound complex. The mass photometry results, shown as a scatter plot and corresponding histogram, reveal the presence of fully and partially assembled species, in agreement with the ns-EM results. (From Sonn-Segev et al. (2020))
The growing recognition of mass photometry as a valuable pre-cryo-EM screening tool is reflected in its widespread adoption across major structural biology facilities. At these institutions, mass photometry is now part of the standard toolkit for evaluating sample suitability before freezing—underscoring its reliability, ease-of-use and value in delivering rapid insights. As more research institutions adopt mass photometry to de-risk early stages of sample preparation, it is fast becoming a core technology in cryo-EM.
Cryo-EM 101, an online training resource created with support from the NIH Common Fund CryoEM Initiative, recognizes mass photometry’s speed and low sample requirements, which it says make it “a desirable technique to screen different buffer conditions before proceeding to negative-stain EM and cryo-EM.” Detailing how mass photometry can be useful for assessing particle quality in cryo-EM workflows, it says mass photometry “can provide information that is not otherwise accessible with other instruments and can streamline the sample characterization process before committing additional resources to cryo-EM.”
Mass photometry is helping researchers optimize buffer conditions and ensure sample integrity before committing precious time and resources to cryo-EM grid preparation. We next explore several recent publications that showcase how researchers are leveraging mass photometry to optimize sample screening, verify structural integrity and improve outcomes across a range of cryo-EM applications.
Membrane proteins remain some of the most technically demanding targets for cryo-EM. Their amphipathic nature makes them highly sensitive to purification and buffer conditions, particularly the choice of detergent or stabilizing agent. Even when purified, membrane proteins are often prone to aggregation, dissociation or conformational instability. Mass photometry can, however, assess samples membrane proteins in membrane mimetics – including detergents and nanodiscs. It can be employed to rapidly and sensitively assess sample homogeneity and oligomeric state to inform optimal buffer conditions.
In a 2024 study (Jiko et al., 2024), researchers explored the impact of different detergents on membrane protein stability in the context of cryo-EM. While differential scanning fluorimetry (DSF) and DLS were used to compare LMNG and a novel detergent, NDT-C11, mass photometry was employed separately to assess the oligomeric state and homogeneity of the membrane protein. These combined biophysical techniques helped the team identify conditions that maintained the protein in a stable, monodisperse state—laying the groundwork for more effective cryo-EM grid preparation.
Accurately determining the oligomeric state of protein complexes is critical in cryo-EM workflows, especially when a protein’s function depends on specific structural assemblies. Samples that are partially dissociated or contain mixed populations can complicate data interpretation and reduce the chances of achieving high-resolution reconstructions. Mass photometry has become an important tool for resolving these challenges.
In a study of the mitochondrial chaperone Skd3 (Gupta et al., 2023), mass photometry was used to investigate how different nucleotide states and mutations influenced the assembly of hexameric and dodecameric forms. These oligomeric transitions were consistent with a proposed mechanism, in which substrate engagement triggers structural rearrangement and activation. As such, mass photometry helped clarify how conformational states observed in cryo-EM relate to behavior in solution.
In a recent structural study of Escherichia coli exonuclease VII (Liu et al., 2024), mass photometry was employed to monitor how various nucleic acid substrates affected the stability of the multi-subunit complex. The authors found that certain substrate forms induced partial disassembly, supporting the hypothesis that exonuclease VII is autoinhibited in its apo form and activated upon substrate binding. This biochemical insight complemented the cryo-EM analysis by explaining conformational variability.
In another research paper (McGregor et al., 2023), researchers studying the mitochondrial Complex I assembly (MCIA) complex used mass photometry to monitor subunit interactions and track the formation of intermediate assemblies under different buffer conditions. This insight was essential for capturing relevant structural states involved in oxidative phosphorylation and neurodegenerative disease mechanisms.
In a broader demonstration of mass photometry’s utility in nucleoprotein structural biology (Sonn-Segev et al., 2020), the technique was used to evaluate the composition and conformational heterogeneity of large assemblies like cohesin and the nuclear pore complex (Figure 5). Mass photometry enabled the researchers to identify intact vs. dissociated subcomplexes, assess sample quality in real time, and benchmark MP against ns-EM. Their results confirmed that mass photometry could confirm the integrity of complexes, which was not possible with ns-EM due to the complexes’ structural flexibility.
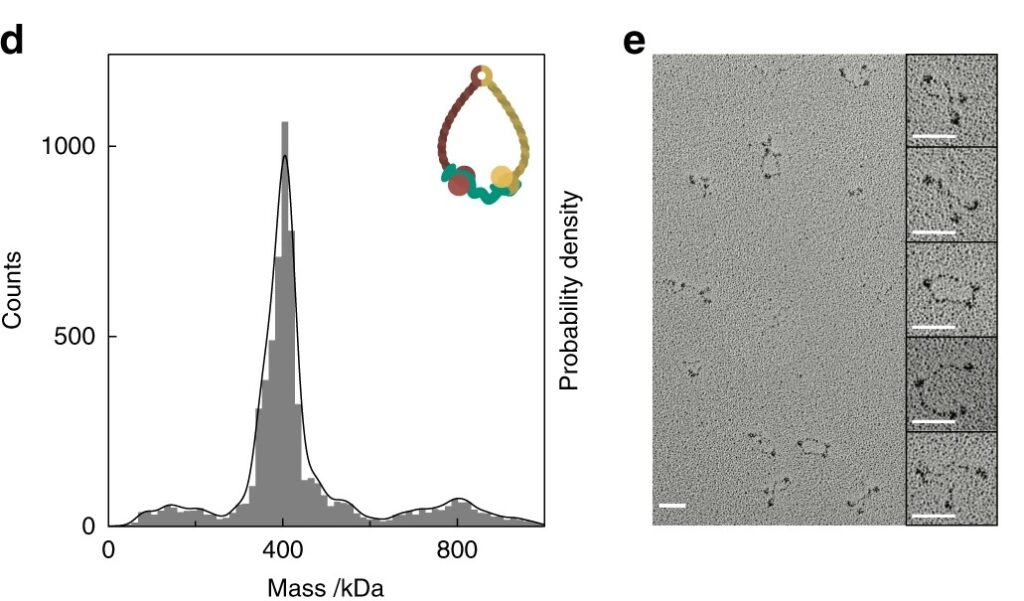
Figure 5: Mass photometry vs. nsEM. Mass photometry (left), but not ns-EM (right), readily characterized a sample of cohesin consisting of flexible coiled coils with variable conformations. Mass photometry reported that the sample contained mainly a species of 410 kDa, as expected. (Figure from Sonn-Segev et al. (2020)).
Together, these examples highlight how mass photometry supports mechanistic insights and complements cryo-EM by revealing the dynamic behaviour and composition of complex protein assemblies in solution.
Protein–DNA and protein–RNA assemblies often present unique challenges for cryo-EM, particularly due to their complexity, frequent heterogeneity, flexible regions and sensitivity to buffer conditions. Ensuring the stoichiometry and integrity of these complexes prior to vitrification is essential, especially when attempting to capture transient or asymmetric assemblies. Mass photometry has proven to be a valuable tool in this space, as it can be used to characterize samples containing proteins as well as nucleic acids.
In a study of the Hermes DNA transposase (Lannes et al., 2023, mass photometry was used to confirm the stoichiometry and assembly state of the protein–DNA complex before freezing. The transposase forms a higher-order oligomer that binds DNA asymmetrically—a structural feature critical to its biological function. By pre-screening samples with mass photometry, the researchers were able to ensure that the samples undergoing cryo-EM analysis were homogeneous samples with complexes in the desired state, thereby maximizing the success of that analysis.
Overall, mass photometry supports a diverse range of cryo-EM applications—including ATPases, exonucleases, membrane complexes and DNA transposases—highlighting its growing role in streamlining and de-risking cryo-EM workflows.
Whether you’re working with challenging targets or looking to scale-up research, integrating mass photometry into your pre-screening pipeline is a practical, proven step toward cryo-EM success.
Discover how mass photometry could empower your cryo-EM research. Contact us today.
Chua, E.Y.D., Mendez, J.H., Rapp, M., Ilca, S.L., Tan, Y.Z., Maruthi, K., Kuang, H., Zimanyi, C.M., Cheng, A., Eng, E.T., Noble, A.J., Potter, C.S., Carragher, B., 2022. Better, Faster, Cheaper: Recent Advances in Cryo-Electron Microscopy. Annu Rev Biochem 91, 1–32. https://doi.org/10.1146/ANNUREV-BIOCHEM-032620-110705
Gupta, A., Lentzsch, A.M., Siegel, A., Yu, Z., Chio, U.S., Cheng, Y., Shan, S.O., 2023. Dodecamer assembly of a metazoan AAA+ chaperone couples substrate extraction to refolding. Sci Adv 9, eadf5336. https://doi.org/10.1126/SCIADV.ADF5336
Jiko, C., Li, J., Moon, Y., Tanaka, Y., Gopalasingam, C.C., Shigematsu, H., Chae, P.S., Kurisu, G., Gerle, C., 2024. NDT-C11 as a Viable Novel Detergent for Single Particle Cryo-EM. Chempluschem 89, e202400242. https://doi.org/10.1002/CPLU.202400242
Lannes, L., Furman, C.M., Hickman, A.B., Dyda, F., 2023. Zinc-finger BED domains drive the formation of the active Hermes transpososome by asymmetric DNA binding. Nature Communications 2023 14:1 14, 1–18. https://doi.org/10.1038/s41467-023-40210-3
Liu, C., Hauk, G., Yan, Q., Berger, J.M., 2024. Structure of Escherichia coli exonuclease VII. Proc Natl Acad Sci USA 121. https://doi.org/10.1073/PNAS.2319644121
McGregor, L., Acajjaoui, S., Desfosses, A., Saïdi, M., Bacia-Verloop, M., Schwarz, J.J., Juyoux, P., von Velsen, J., Bowler, M.W., McCarthy, A.A., Kandiah, E., Gutsche, I., Soler-Lopez, M., 2023. The assembly of the Mitochondrial Complex I Assembly complex uncovers a redox pathway coordination. Nature Communications 2023 14:1 14, 1–17. https://doi.org/10.1038/s41467-023-43865-0
Niebling, S., Veith, K., Vollmer, B., Lizarrondo, J., Burastero, O., Schiller, J., Struve García, A., Lewe, P., Seuring, C., Witt, S., García-Alai, M., 2022. Biophysical Screening Pipeline for Cryo-EM Grid Preparation of Membrane Proteins. Front Mol Biosci 9, 882288. https://doi.org/10.3389/FMOLB.2022.882288
Sonn-Segev, A., Belacic, K., Bodrug, T., Young, G., VanderLinden, R.T., Schulman, B.A., Schimpf, J., Friedrich, T., Dip, P.V., Schwartz, T.U., Bauer, B., Peters, J.M., Struwe, W.B., Benesch, J.L.P., Brown, N.G., Haselbach, D., Kukura, P., 2020. Quantifying the heterogeneity of macromolecular machines by mass photometry. Nature Communications 2020 11:1 11, 1–10. https://doi.org/10.1038/s41467-020-15642-w
Vostal, L. E. 2025. Need for speed: Mass photometry as a sample analysis tool for structural studies. Structure 33, 994–996. https://doi.org/10.1016/j.str.2025.04.014