
The remarkable evolution of mass photometry was celebrated at the annual mass photometry user summit. From prototype to commercially available instrument used in hundreds of laboratories, this innovative technology was on display at the third annual scientific meeting of this event – dedicated to mass photometry users and hosted by Refeyn. The event took place at Wylie Conference Center on May 22 in Beverly, Massachusetts, a coastal suburb of Boston, USA.

Refeyn is delighted to announce the appointment of Fiona Coats as Chief Marketing Officer, effective from June, 2024. Forming part of Refeyn’s executive team, Fiona will bring with her a wealth of experience from over 25 years in the biotechnology sector.

New US customer interaction center opens in Massachusetts’ Waltham Biotech Hub with 3rd annual Mass Photometry Summit set to highlight latest advances

ABL, an Institut Mérieux Company, is a distinguished contract development and manufacturing organization (CDMO) providing GMP viral vectors from early stage to market.

New macro mass photometer can assess the stability, quality, and purity of adenovirus and lentivirus vectors within just minutes
Refeyn is pleased to announce the expansion of the consumables portfolio for mass photometry. Refeyn has created a new product line of mass reference standards, MassFerence, which help streamline sample characterization with mass photometry.

Neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, both involve an accumulation of protein aggregates [1],[2]. Monitoring and studying small oligomers, which are present in the early stages of disease, can be challenging, due to their transient and heterogeneous nature [1].
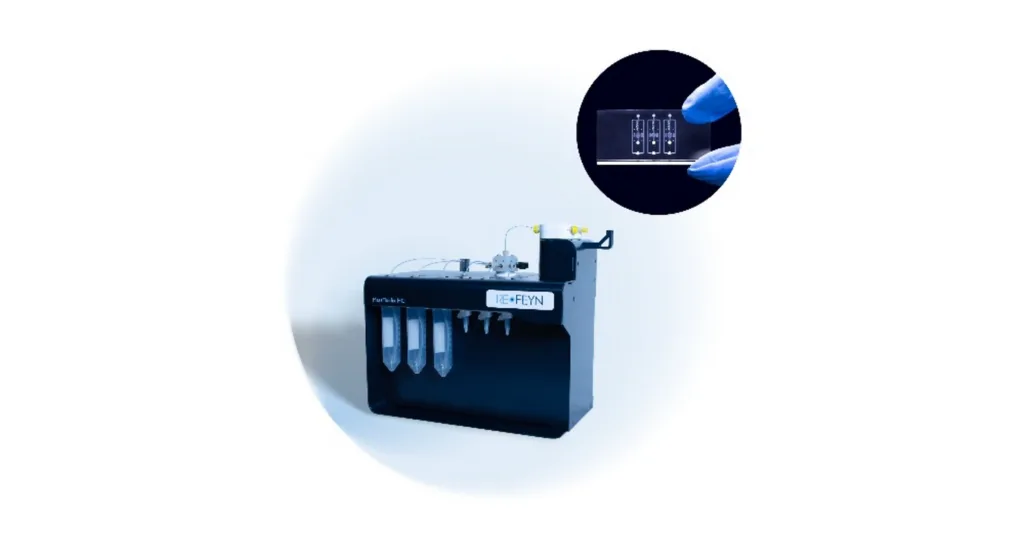
New microfluidic system enhances protein analysis workflows for the study of weak protein-protein interactions by mass photometry

Refeyn, a leading Life Science instrumentation company pioneering mass photometry, announced today that Gerry Mackay has been appointed the new CEO of the company effective January 1st, 2024.

Lipoprotein lipase (LPL) has long been a focal point in the study of lipid metabolism. Its role in releasing free fatty acids from triglycerides, allowing their transport across cell membranes, is vital for energy production, while dysregulation of this enzyme has been linked to cardiovascular disease [1].










