Mass photometry and macro mass photometry are powerful bioanalytical techniques that are being adopted across the life sciences. From academic researchers to CDMOs and leading biopharma companies, many have harnessed mass photometry technologies to gain vital information about their samples. Could they make a difference for you, too? Read on to learn about key applications and comparisons to other technologies.
Mass photometry technologies are based on the principle of interferometric scattering microscopy. They measure the interference between the light reflected by a glass surface (e.g. a coverslip) and the light scattered by particles in contact with the surface. Mass photometry and macro mass photometry each work slightly differently but, at their core, what they both offer is simple:
They report the different particle populations in your sample and their relative proportions in solution, with no labels or complex prep, and using up little of your valuable time or sample.
The information provided by mass photometry technologies can be valuable in a very broad range of situations, as it can readily detect impurities, oligomerization, capsid loading, aggregates or complexes with different stoichiometries. There are a number of applications where the unique strengths of mass photometry technologies really shine. Let’s look at several use cases and what mass photometry technologies offer for each, and how they overcome challenges that other techniques struggle to address.
The study of protein-protein interactions can be challenging, as they often involve multiple components, complex, dynamic equilibria or a high sensitivity to experimental conditions.
Why use mass photometry?
Mass photometry is a powerful tool for analyzing protein-protein interactions. Unlike techniques like size-exclusion chromatography (SEC) and biolayer interferometry (BLI), it works in solution with no columns or immobilization.
Mass photometry provides single-particle information rather than the bulk information reported by techniques such as dynamic light scattering (DLS), providing high-resolution information on complex formation, oligomerization behavior, stoichiometry and binding affinity.
The preparation for mass photometry measurements requires only a simple dilution to nanomolar concentrations. This captures the behavior of proteins in native-like concentrations and avoids the denaturation induced by SDS-PAGE. If measurements at higher concentrations are needed, the MassFluidix™ HC microfluidics add-on can be used to measure samples in the micromolar concentration range.
Finally, as mass photometry measurements are fast and consume little sample, it is easy to quickly test different experimental conditions.
Case study
This paper explains in detail how to use mass photometry to determine the mass distribution of a protein sample, revealing the complexes formed and relative abundance of each species, and, accordingly, the dissociation constant(s) (KD) of interactions.
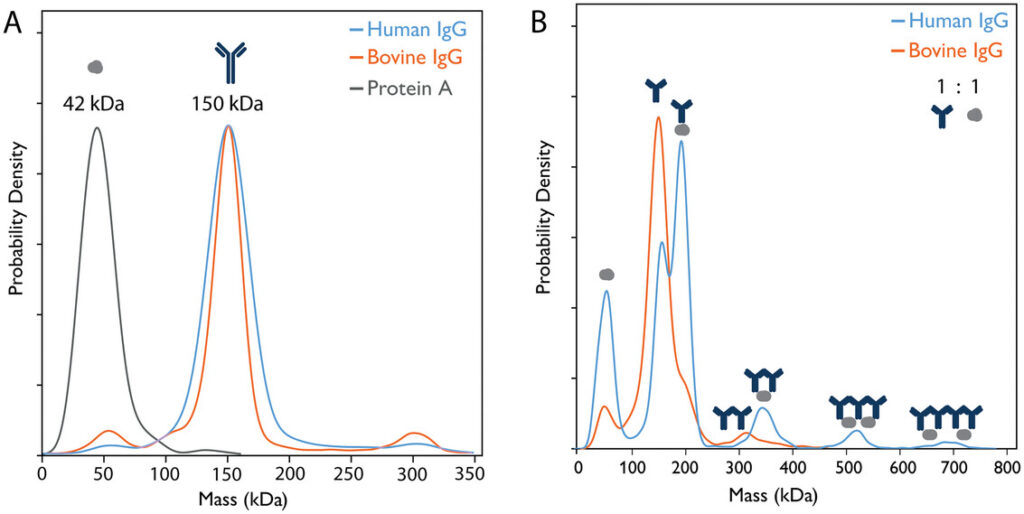
Mass photometry measurement of interactions between protein A, human IgG and Bovine IgG. Panel A shows the overlapped individual measurements for each species, panel B shows overlapped measurements of 1:1 mixes of protein A and human IgG or bovine IgG. Mass photometry detects each individual species and the multiple human IgG-protein A complexes. As expected, bovine IgG does not bind to protein A and shows a small number of aggregates. Figure 1 from Kofinova et al. (2024).
Cutting-edge analytical techniques for structural biology such as cryo-EM are amazingly powerful, revealing intricate details about protein structure and interactions. However, instrument time is very valuable, and sample preparation is tricky, so it is critical to make sure proteins are monodisperse and in their desired oligomeric state before the measurement.
Why use mass photometry?
Mass photometry reports purity, stability and oligomeric states of samples with low time and sample volume requirements. The information it provides is on par with negative-stain electron microscopy (nsEM), and more detailed than techniques like SEC, DLS and SDS-PAGE. In addition, mass photometry requires very little time and sample, making it an ideal way to check samples before using precious cryo-EM time.
Case study
Protein quaternary structures in solution are a mixture of multiple forms (Marciano et al., 2022)
The authors of this paper evaluated the oligomeric state of 17 different bacterial proteins across a broad range of protein concentrations and solutions by native mass spectrometry, mass photometry, SEC, and small-angle X-ray scattering (SAXS), finding that most exhibit several oligomeric states at the same time. For approximately half of the proteins, the predicted oligomeric forms described in publicly available databases underestimated the complexity of protein quaternary structures in solution.
mRNA is the active payload for many gene therapies, but therapeutic mRNA sequences are difficult and expensive to produce. They require careful characterization to ensure the final product is safe and effective. As mRNA molecules are highly charged and quite large compared to most biologics, their analysis can be challenging.
Why use mass photometry?
Mass photometry can readily measure mRNA molecules and report on key attributes of the sample. As it reports the mass distribution of particles in the sample, it provides an overview of the lengths of the mRNA molecules present. A key advantage is that it can be used for mRNA of up to ~10,000 bases in length or more, depending on characteristics of each sample and buffer. A mass photometry measurement quickly shows if the transcript is intact and detects impurities such as aggregation or dsRNA. As it is fast, consumes little sample and has low operating costs, mass photometry enables frequent, at-line characterization of mRNA products without slowing down critical processes.
Case study
This paper reports on the use of mass photometry to directly measure RNA length, showing that it can address some of the current challenges in mRNA analytics and can serve as a useful orthogonal technique.
The discovery, development and production of antibody-based therapeutics require careful, repeated bioanalytical characterization of both the antibody candidates and their target antigens. New antibody modalities come with their own analytical challenges, as their complex interactions with multiple antigens make them harder to characterize with techniques like nanoparticle tracking analysis (NTA) or biolayer interferometry (BLI).
Why use mass photometry?
Mass photometry offers key advantages that make it an invaluable tool to derisk antibody development and production. It provides data comparable to gold-standard methods such as SEC but with minimal acquisition time (one minute) and sample consumption (15 – 30 ng), making it ideal for repeated, at-line analyses without slowing down processes.
As a single-particle technique, mass photometry detects all the populations in a sample within its mass range, even those present in small quantities, allowing it to readily detect aggregation. The working concentrations of mass photometry are ideal to study aggregation behavior in relevant conditions, such as the low concentrations found inside IV bags. A microfluidics add-on, MassFluidix HC, makes it possible to measure samples at micromolar concentrations, enabling comparison to SEC.
A mass photometry measurement detects, in a single measurement, free antigens and antibodies as well as their complexes, and quantifies their abundance. This information can be used to assess antigen quality and determine antibodies’ preferred stoichiometries and binding affinities. Mass photometry is modality-agnostic and can also be applied to more complex samples (including bispecifics and multispecifics) with no additional preparation or method development. Combined with its low time and sample consumption, the strengths of mass photometry make it ideal for work with large antibody libraries and new modalities.
Case study
This study (currently still a preprint) used mass photometry and kinetic modelling to analyze the interactions between the bispecific antibody ivonescimab and its targets (VEGF and PD-1), quantifying the complexes formed and their affinities. They confirmed that VEGF induced ivonescimab to oligomerize and revealed dimers as the most stable structure – not higher-order structures, as previously thought.
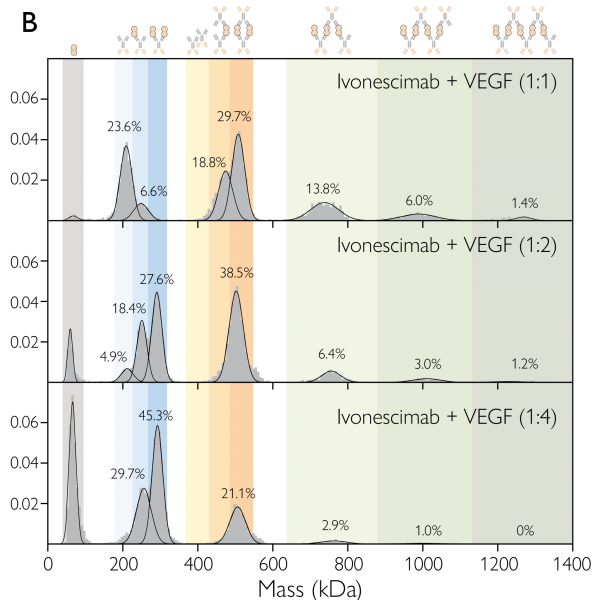
Mass photometry analysis of ivonescimab:VEGF interactions. The mass photometry histograms show ivonescimab:VEGF complex formation for mixtures with 1:1, 1:2 and 1:4 antibody:antigen concentration ratios, at equilibrium, after 40 min incubation. Mass photometry detects and quantifies the multiple antibody-antigen complexes (see schematics above the histograms), helping understand how ivonescimab interacts with its targets. Figure 2B from Jajcanin Jozic et al. (2025).
To study membrane proteins, they need to be embedded in membrane mimetics to preserve the structure of their hydrophobic domains. Developing the right protocol for membrane protein preparation can be challenging, and it often comes down to trial and error.
Why use mass photometry?
Unlike other techniques, mass photometry is compatible with most membrane mimetics, and its low sample and time requirements are amenable to repeated analysis while developing protocols.
Case study
Mass Photometry of Membrane Proteins (Olerinyova et al., 2021)
This paper demonstrates how mass photometry can be used to study proteins solubilized using different types of membrane mimetics, providing information on the heterogeneity of samples and the functional state of the studied proteins.
Some research (e.g. the study of DNA repair mechanisms) requires characterizing protein-DNA interactions. This is not straightforward, since protein-DNA complexes can be very heterogeneous in size and have variable charge-to-mass ratios due to variation in the number of proteins bound specifically and non-specifically to DNA.
Why use mass photometry?
Mass photometry enables the detection of protein-DNA and protein-protein interactions in solution without the need for labels or tags and without disruptive sample preparation. This is useful for studying the dynamic assembly and disassembly of protein-DNA complexes, which may involve transient interactions between multiple proteins.
Case study
This structural study used mass photometry along with other techniques and structure modeling to study the stimulation of S. cerevisiae Dna2 by RPA. They found that the large RPA subunit Rfa1 alone can promote the Dna2 nuclease activity and that different domains of Rfa1 regulate Dna2 recruitment, and its nuclease and helicase activities.
Producing safe and effective AAV-based therapeutics requires screening samples for impurities that may impact safety and immunogenicity. One concern when producing AAV-based products is the presence of impurities in the form of capsids that do not contain the recombinant genomic payload, carry only a partial copy, or are overfull.
Why use mass photometry?
Current techniques available to characterize AAV capsid loading suffer from several disadvantages. Analytical ultracentrifugation (AUC) provides excellent resolution, but takes time, requires large amounts of sample and often needs to be outsourced. Other techniques like qPCR/ELISA or SEC-MALS have disadvantages including poor resolution and serotype specificity.
Mass photometry reports on multiple relevant attributes of AAV samples. Thanks to its single-molecule mass measurements, it readily quantifies the proportions of empty/full/partially filled capsids and detects overfull capsids and aggregation. In addition genome size can be estimated from the difference in mass between full and empty capsids. The information provided by mass photometry is on par with AUC, but its throughput, mass consumption, operating costs and instrument footprint are drastically lower, allowing for frequent, in-house analyses.
With a short cleanup step, mass photometry can also analyze AAV samples after upstream processing. In addition, Refeyn offers software that supports analysis in GMP-compliant environements to provide fast, informative analytics that support AAV-based therapeutic manufacturing.
Case study
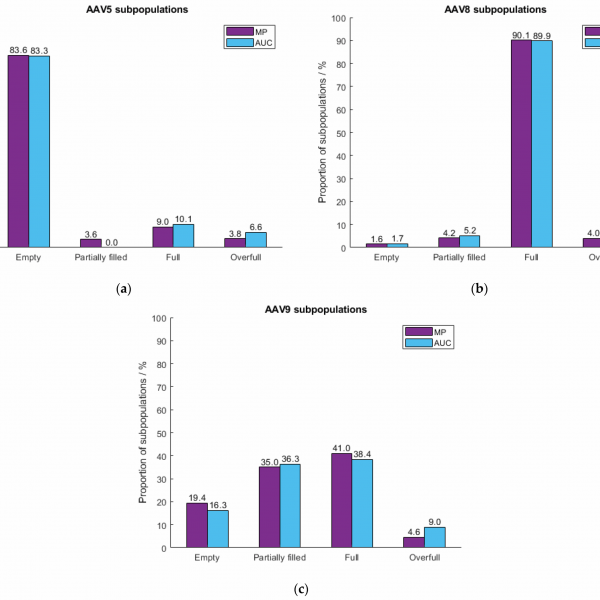
A comparison of mass photometry and AUC measurements of different AAV serotypes. This study showed that mass photometry results are comparable to those from AUC, while requiring much less time and sample. Figure 6 from Wagner et al. (2023).
The safety and efficacy of therapeutics using vectors such as adenoviruses (AdVs), lentiviruses (LVVs) and virus-like particles (VLPs) require careful characterization to make sure the final product is pure and stable.
Why use macro mass photometry?
Among the challenges of current techniques are difficulties detecting and distinguishing different populations in the sample, including the critically important empty and full vectors. Those able to distinguish populations suffer from long measurement times, high operating costs or high sample consumption.
The single-particle, multiparametric analyses performed by macro mass photometry distinguish and quantify the different populations in the sample. This includes not only empty and full vectors, but other relevant components of the sample like aggregates, process-related impurities and helper virus populations in the case of AdV. Moreover, macro mass photometry delivers all this information in minutes, with minimal sample consumption and very low operating costs. This makes macro mass photometry ideal for frequent, in-house analyses of viral vector samples, speeding up process development and derisking production.
Case study
Quantifying adenovirus packaging with macro mass photometry
This application note from Refeyn – created in collaboration with the Jenner Institute at the University of Oxford – shows how macro mass photometry can be used to quickly evaluate adenovirus packaging. It also shows that macro mass photometry can distinguish adenoviral vectors from helper virus populations. Results are compared to expected values as well as to results from orthogonal techniques, including AUC and nsEM.
Think mass photometry may be right for you? For more information, do not hesitate to contact us.
To learn more, check out these comprehensive resources:
Mass photometry handbook – Refeyn
Curious how mass photometry delivers accurate results – fast? Whether you’re new to the technique or looking to get more from your experiments, our handbook Understanding Mass Photometry is your essential guide.
Discover how it works and how to maximize its potential. Find out how it compares to other light scattering techniques, how it’s being used across the life sciences, and what others have to say about this unique technology.
Why is mass photometry appearing in over 1300 scientific studies and being adopted by Pfizer, GSK and Astra Zeneca? In this webinar, a Refeyn expert explains what it is that makes mass photometry such a powerful and versatile technique.