Process insights to streamline lentivirus production
Macro mass photometry enables comprehensive analysis of lentiviral populations and associated impurities
Rapid, high-resolution analysis of lentiviral vector process samples
Lentiviral vectors (LVV) are central to advancing gene therapies, yet conventional analytical tools fail to quickly deliver the actionable information needed to inform process decisions and improve optimization. At Refeyn, our cutting-edge macro mass photometry (MMP) technology provides high-resolution insights into lentiviral vector samples – quickly revealing impurities and heterogeneity, even in crude samples.
Key pain points experienced by scientists and engineers developing lentiviral therapies:
Complex purity assessment:
The heterogeneity of LVV samples makes them difficult to fully characterize, yet purity assessment is critical to ensure safety and efficacy.
Limited early-stage visibility
A lack of effective tools for upstream assessment makes it challenging to identify and address problems before making decisions about downstream investment.
Slow, sample-heavy analytics
To enable robust process monitoring and regulatory compliance, there is a need for fast, informative analytical techniques that consume little sample.
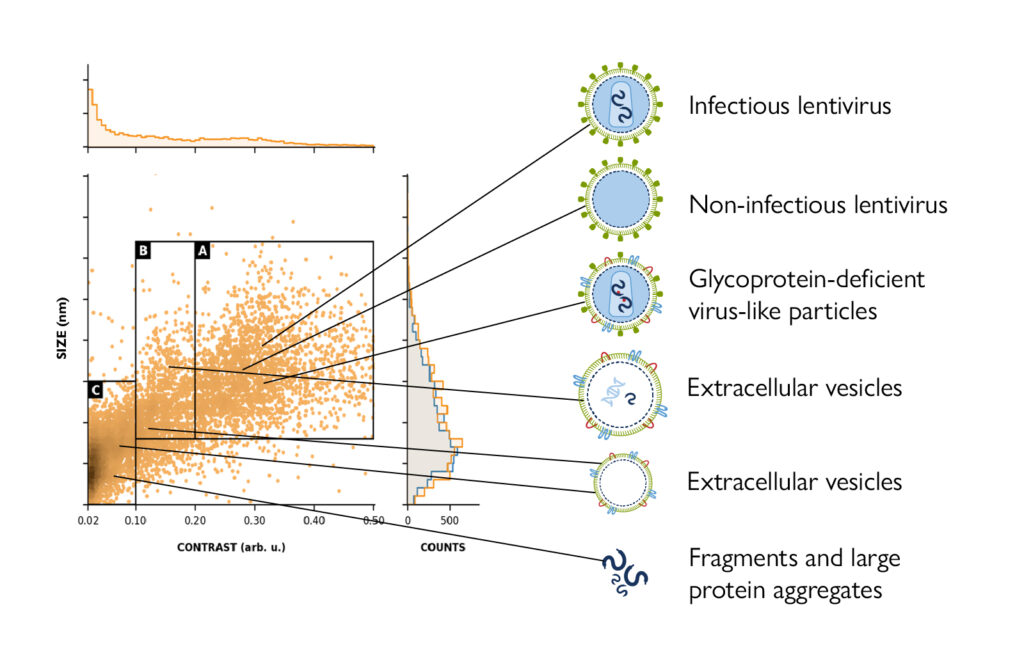
Figure 1. MMP captures the heterogeneity of LVV samples. LVV samples contain a mix of impurities as well as intact, infectious particles, which many techniques struggle to characterize. MMP precisely distinguishes heterogeneous populations – providing accurate, quantitative purity assessment by measuring particle size and contrast (a proxy for mass).
Challenges
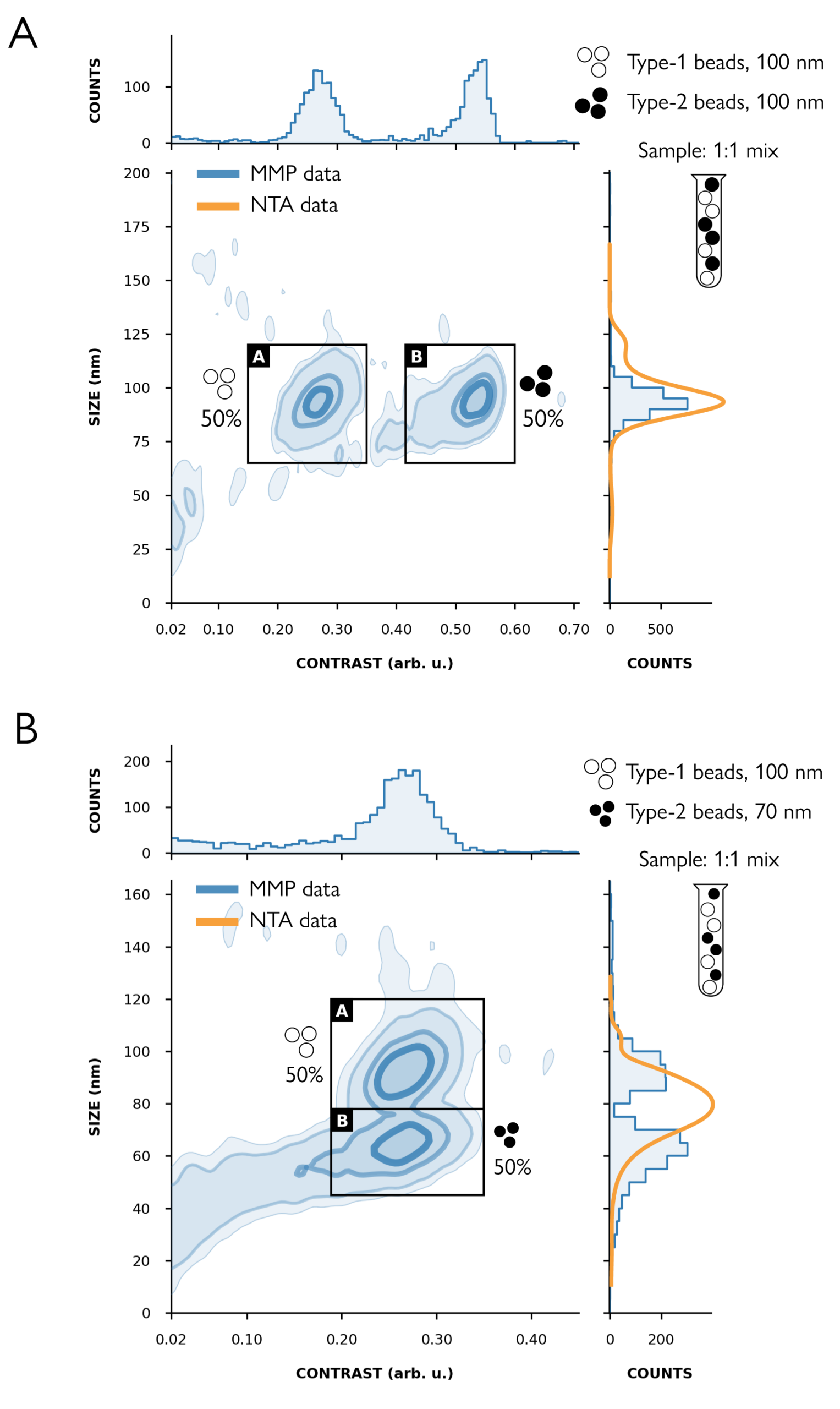
Figure 2. MMP clearly resolves populations that cannot be resolved using NTA – as illustrated with 1:1 mixtures of beads. A) Both sets of beads are 100 nm in diameter but they have different contrasts (mass proxy): B) The beads have different sizes (70 nm, 100 nm) but similar contrast. Measured on the Karitro™* macro mass photometer.
Insufficient analytical resolution to accurately profile impurities
LVV preparations are inherently heterogeneous. Alongside intact, genome-containing infectious particles, they frequently include defective or partially assembled particles, non-infectious forms, extracellular vesicles, cellular debris, and aggregates.
Inadequate characterization of product quality can undermine both efficacy and safety, ultimately diminishing therapeutic benefit and increasing the risk of immunogenicity. In addition, elevated impurity levels can adversely affect downstream processing performance, leading to reduced recovery, lower yields, and compromised transduction efficiency.
MMP is an advanced single-particle analytical technology that delivers a highly informative, multiparametric readout. It offers precise discrimination of heterogeneous particle populations and provides an accurate, quantitative assessment of sample purity. MMP resolves the distinct components within complex mixtures by simultaneously measuring particle size and contrast (a proxy for mass).
Although many viral vectors have similar physical sizes, their masses vary depending on factors such as the size and composition of their genome cargo, cargo-packing efficiency, and, to a lesser extent, their structural protein and lipid envelope composition. MMP enables the clear identification and quantification of particle populations with different sizes and contrasts – such as vectors carrying different cargo as well as impurities and aggregates. Compared to other methods, such as nanoparticle tracking analysis (NTA), MMP offers deeper insight into vector and sample composition, supporting more rigorous control of product quality.
Reliable purity analysis
In upstream analysis of LVV preparations, impurities and overall productivity are often insufficiently characterized. Early assessment of sample purity is critical, as impurity profiles directly influence the efficiency and robustness of subsequent purification steps. This, in turn, affects the developability of the vector, determining whether a process can be reliably scaled, consistently manufactured, and translated into a viable clinical or commercial product. Poor developability often leads to higher costs, delays, and manufacturing risks.
LVV are frequently analyzed only at the end of their production process, so there is little information available to guide earlier intervention. Incorporating upstream sample analysis into the workflow enables tighter process controls and allows critical decisions to be made sooner – minimizing downstream losses, reducing resource expenditure, and accelerating overall production timelines.
There is therefore a growing need for analytical approaches that deliver reliable, quality-indicating data during upstream process development to support timely go/no-go decisions and prevent costly downstream delays. Optimization of transfection conditions, as well as improvements in yield and purity, can be significantly enhanced by employing multi-attribute analytical methods such as MMP.
Conventional assays that focus solely on titer typically provide only a single parameter, leaving substantial gaps in understanding the true composition and quality of the material. In contrast, MMP offers a richer, multiparametric dataset, enabling a more comprehensive evaluation of vector quality early in development and thereby strengthening overall process developability.
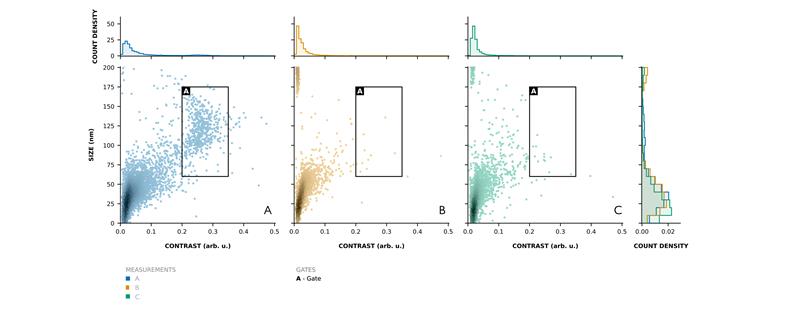
Figure 3. MMP distinguishes LVV particles from impurities without any labelling or sample pretreatment. MMP analysis of LVV (A), negative control (B) and non-infectious (C) particles shows MMP can distinguish impurities from larger, heavier LVV particles based on their lower contrast (mass proxy) and smaller size. Measured on the KaritroMP.
Rapid, high-resolution measurements
Inadequate process monitoring can create major challenges during scale-up and tech transfer, and introduce variability across production batches. It also increases the risk of regulatory rejection due to limited oversight of critical process parameters.
Effective process monitoring requires rapid measurements that provide immediate, actionable feedback on sample quality, unit operation performance, and any deviations that arise. However, current analytical methods typically allow evaluation of process efficiency and yield only after a run is complete.
MMP, a true multi-attribute analytical technique, provides earlier, real-time insight into multiple quality-defining characteristics – enabling effective process monitoring. It also significantly accelerates analysis of multiple fractions, offering rapid, multi-attribute insight across downstream fractions, upstream material, stability studies, and process comparability.
Instead of waiting days for conventional assays, meaningful data can be obtained within hours. Automated acquisition further streamlines workflows, delivering results for 14 samples in around 90 minutes – enabling faster decisions, earlier optimization, and greater confidence in process performance.
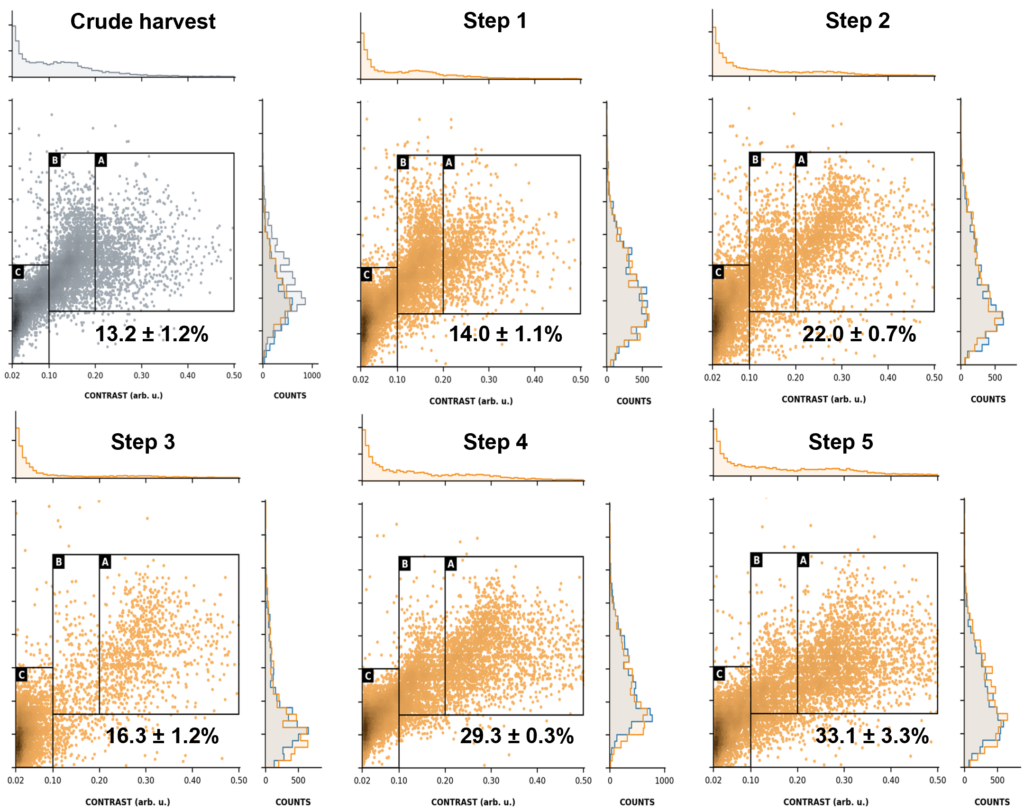
Figure 4. MMP provides clear insights into sample composition throughout processing. LVV samples collected in upstream production and after five sequential purification steps were analyzed by MMP using gated regions that resolve: LVVs (Gate A), EVs (B), and cell debris or smaller vesicles including degraded EVs and LVVs (C).
High sample volume requirements
Sample volume requirements across standard analytical assays are often substantial. This is especially problematic for lentiviral vector LVV programs, where material is expensive, limited, and increasingly scarce at later, highly concentrated stages. Preserving material is therefore essential, while still ensuring enough volume remains for critical characterization assays.
MMP offers a streamlined, yet highly effective solution. Each analysis requires only 5 µL of sample – even after dilution – with no lengthy pretreatment. Samples from any stage of the process, from crude harvest to purified fractions, can be tested immediately, enabling faster, lower-volume decision-making throughout the workflow.
Beyond minimal sample consumption, MMP is also highly cost-efficient: it uses at least 20× less material than ELISA and, at under $10 per measurement, is roughly 7× more cost-effective. This combination makes MMP an ideal choice for high-value, volume-constrained LVV development.
Mass photometry solutions for lentivirus
Refeyn KaritroMP
The KaritroMP is a powerful macro mass photometer designed for rapid, label-free characterization of large viral vectors.

It delivers unique insights into LVV preparations by simultaneously measuring particle size and contrast (a proxy for mass) – enabling rapid assessment of vector purity, composition, and yield. This makes it an ideal tool for fast, label-free analysis.
Its core features directly address several key challenges in LVV characterization:
Multiparametric analysis
Provides both size and contrast (mass proxy), allowing differentiation of intact vectors from impurities and partially assembled particles.
Rapid analysis
Automated acquisition enables analysis of up to 14 samples in just 90 minutes, accelerating decision-making across the workflow – from upstream to post-purification.
Single-molecule precision
High-resolution, label-free detection ensures accurate measurement of individual LVV particles and associated impurities, delivering unprecedented clarity in sample quality assessment.
MassGlass™ KV Slides
A high-quality measurement surface designed for use with the KaritroMP instrument.

MassGlass™ KV slides provide an out-of-the-box, ready-to-use solution that delivers reliable and consistent performance for viral vector measurements.
Key advantages include:
Ready-to-use format
Streamlines setup and accelerates analysis.
Guaranteed performance
Ensures high-quality, reproducible measurements every time.
User-friendly design
Easy to handle and intuitive, enabling seamless operation within the system.
Complete software solution: AcquireMPK and DiscoverMPK
Integrated acquisition and analysis software packages that provide an intuitive, easy-to-use solution for macro mass photometry workflows.
Designed to streamline both data capture and interpretation, this software suite enables rapid measurements, providing clear user guidance and powerful analytical capabilities. It was built specifically for researchers working with complex samples on the KaritroMP.
Its core features support fast, confident decision-making:
User-Friendly Interface
Embedded video guidance supports the user through every step of the analysis, ensuring smooth and error-free operation.
Integrated, automated calibration
Built-in calibration routines ensure that every measurement begins with an accurate, reliable setup for high-quality data.
Instant multiparametric reporting
High-fidelity data processing with smart visualization tools quickly deliver rich, interpretable results – providing the clarity needed for rapid, informed decisions.
Resources
Data-Driven LVV Process Development Through Macro Mass Photometry
This on-demand webinar explores how MMP delivers rapid, multiparametric analysis to accelerate optimization across all stages of LVV development. Learn how this innovative approach overcomes traditional analytical bottlenecks and provides real-time, data-driven insights.
Resolving heterogeneous viral vector populations with the KaritroMP
MMP reveals and quantifies diverse particle populations in complex AdV and LVV samples. Using the Karitro™ macro mass photometer, in this tech note we showcase multiparametric measurement principles and benchmark performance against nanoparticle tracking analysis for deeper sample insight.
Frequently asked questions
What is MMP and how does it work?
MMP is a bioanalytic technology that analyzes large viral vectors, such as LVV’s, at the single-particle level. It measures each particle’s size and contrast (a proxy for mass), allowing users to assess vector integrity, purity, heterogeneity, and overall product quality without labels or complex preparation.
How does MMP measure LVV size and purity?
By quantifying size and mass-related contrast, it can separate intact LVVs from partially assembled particles, empty-like particles, debris, and aggregates, giving a true representation of sample purity.
Do I need an experimental control for LVV analysis with macro mass photometry?
A negative control, typically a mock transfection sample, is required when analyzing LVVs with MMP. This control provides a crucial baseline for distinguishing vector-specific particles from background components originating from cells, media, or process impurities.
How much sample volume does MMP require for lentiviral vector LVV analysis?
The KaritroMP macro mass photometer provides results within minutes, and its automated acquisition mode can analyze up to 14 samples in approximately 90 minutes. This enables rapid feedback during development, optimization, or troubleshooting.
Can MMP detect impurities and non-intact lentiviral vector particles?
Yes. MMP distinguishes impurities based on size, mass-related contrast, and population distribution. It can detect non-intact particles, partially packaged vectors, exosomes, and debris – providing clarity that conventional bulk assays do not offer.
How fast can MMP analyze lentiviral vector samples and deliver results?
Yes. Mass photometry works across all antibody modalities, including monoclonals, bispecifics, multispecifics and ADCs. Its native-state, column‑free, label-free measurement ensures reliable characterization of aggregation.
In fact, as mass photometry simply measures molecular mass, it can be applied to a wide range of samples for multiple uses. For example, it can also analyze antibody-antigen binding, characterize mRNA samples and check sample quality prior to analysis by methods such as cryo-EM.
Can MMP analyze crude LVV harvests or only purified material?
MMP is compatible with all stages of the process – including crude harvests, downstream fractions, and purified LVVs.
Can MMP measure lentivirus titer?
MMP provides orthogonal, physical-attribute information (size, mass-related contrast, heterogeneity) and physical titer (feature in development). It complements other analyses, such as functional titer assays – providing insight into why titer or potency might change between batches.
Is MMP suitable for stability and comparability studies with LVVs?
Yes. Because it is single-particle and multiparametric, MMP is well suited to monitoring LVV stability over time, spotting emerging aggregates or fragmented species, and comparing batches, processes, or formulations side by side.
Can AAVs also be analyzed using MMP?
MMP can characterize larger viral vectors, typically in the size range of 40–150 nm, For AAVs, mass photometry is the more appropriate analytical technology. The Samux mass photometer (and SamuxMP Auto) are optimized for AAV characterization.